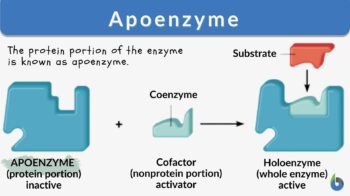
Apoenzyme
n., plural: apoenzymes
[ˌæpəʊˈɛnzaɪm]
Definition: protein component, which together with a cofactor forms a complete enzyme
Table of Contents
Enzymes are biological catalysts that can increase the rate of chemical reactions in living organisms. Enzymes can only function in suitable conditions of specific pH, pressure, and temperature. They are highly specific and effective for each chemical reaction in the body. Enzymes act on substrates by modifying them to give a new product. They also decrease the activation energy required to initiate the reaction so the reaction speed increases. Enzymes remain unchanged during chemical reactions, so they can be reused several times. Simple enzymes such as trypsin, pepsin, and urease are not associated with cofactors but some enzymes are associated with small non-protein molecules known as cofactors and these enzymes are known as conjugate enzymes.
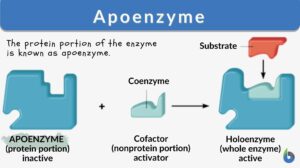
Cofactors may be a prosthetic group or coenzymes. Coenzymes are not tightly bound to the apoenzyme; therefore, they can be easily removed from the active site of an enzyme. Water-soluble vitamins or coenzymes such as vitamin C and Vitamin B, as well as elements like calcium, magnesium, iron, copper, zinc, and potassium act as cofactors. Prosthetic groups are usually loosely bound to the apoenzyme. Cofactors help in carrying out reactions that standard enzymes cannot. Cofactors may be small organic compounds such as flavin or inorganic metal ions. The protein portion of the enzyme is known as apoenzyme.
Apoenzyme Definition
What is apoenzyme? Apoenzyme is the protein part of an enzyme. The non-protein part cofactor together with the protein part apoenzyme forms a holoenzyme. Apoenzymes are important for enzymatic activity since they are responsible for the specificity of enzymes to their substrates. Apoenzymes alone are not active enzymes; they must bind to an organic or inorganic cofactor in order to be activated.
What is the difference between apoenzyme and holoenzyme? After binding to a cofactor, apoenzyme forms a holoenzyme which is an active enzyme and can perform the catalytic activity. Most cofactors are bound tightly to a coenzyme but they are not bound by a covalent bond. However, some prosthetic organic groups such as vitamins or iron might be bound covalently. When the coenzyme is not tightly bound to the apoenzyme, the reaction would be considered a reaction of two substrates with the apoenzyme since the coenzyme and the substrate must bind to the apoenzyme before initiation of the reaction. For example, coenzyme NADH and the pyruvate substrate should be added to the apoenzyme in lactate dehydrogenase in order to perform its catalytic function.
Coenzymes cannot be synthesized inside the body so they must be taken with food in the diet. Therefore, vitamins and minerals are physiologically important since they participate in different enzymatic processes.
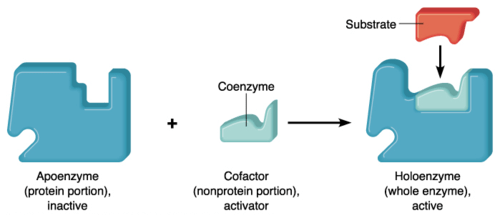
Apoenzyme is the protein component; if bound to a cofactor forms a complete enzyme.
Apoenzyme Examples
What is the nature of Apoenzyme? Apoenzymes are non-dialyzable protein macromolecules that can be destroyed by heat (thermolabile) whereas coenzymes are molecules of small size that cannot be destroyed by heat since coenzymes are not proteins, together they form a holoenzyme. Examples of enzymes consisting of apoenzymes and coenzymes are transferases, oxidoreductases, ligases, and isomerases.
Apoenzyme in DNA polymerase
Apoenzymes are essential for a holoenzyme to function properly. One of the most important holoenzymes is DNA polymerase which is composed of a cofactor and an apoenzyme. DNA polymerase catalyzes DNA formation through the polymerization of deoxyribonucleotides. It contributes to the replication of DNA strands by synthesizing new DNA strands after reading the intact DNA strand. It forms a new complementary strand to the template strand and identical to its partner strand. The prosthetic cofactor of DNA polymerase is a magnesium ion.
Apoenzyme in RNA polymerase
RNA polymerase is another holoenzyme composed of a cofactor and apoenzyme. RNA polymerase uses DNA templates to construct RNA chains in a process known as transcription.
Importance of Apoenzymes
Synthetic cofactors and apoenzymes are being developed in order to produce new holoenzymes with improved activity or new functions.
Apoenzymes bind to coenzymes forming a holoenzyme to catalyze the formation of an active vitamin from the inactive form. Therefore, the deficiency in the activity of any component of the holoenzyme will result in a deficiency in the produced vitamin which might lead to health problems. The apoenzyme is used in measuring the level of B6 by stimulating apoenzyme activity.
Apoenzymes are involved in catalytic reactions, they undergo changes to allow the transformation of the substrate into a product. Cofactors are essential for apoenzymes to perform their catalytic activity. For example, coenzymes can transfer or accept the electrons or hydrogens from or to the substrate in oxidoreductases.
Apoenzymes differ from one holoenzyme to another while catalyzing different reactions, but more than one apoenzyme may be associated with the same coenzyme acting on different substrates.
For example, NAD coenzymes are associated with apoenzymes of glutamate dehydrogenase, lactate dehydrogenase, malate dehydrogenase, and oxidoreductases. The function of the coenzyme NAD is to accept hydrogens liberated from different substrates. Therefore, the apoenzyme protein portion is responsible for the specificity of the holoenzyme as well as the recognition of the substrate-specific for each holoenzyme.
Try to answer the quiz below to check what you have learned so far about apoenzymes.
Further Reading
References
- Berg, J. M., Tymoczko, J. L., & Stryer, L. (2006). Biochemistry (Biochemistry (Berg)).
- Fruk, L., Kuo, C. H., Torres, E., & Niemeyer, C. M. (2009). Apoenzyme reconstitution as a chemical tool for structural enzymology and biotechnology. Angewandte Chemie International Edition, 48(9), 1550-1574.
- Blanco, A., & Blanco, G. (2017). Chapter 8—enzymes. Medical biochemistry, 8, 153-175.
- Cooper, G. M. (2000). The central role of enzymes as biological catalysts. Sinauer Associates.
- Jain, V.K. and Sharma, J.P. (2005). Comprehensive Objective Biology. New Delhi, India: Golden Bells. p.99
- Litwack, Gerald (2018). chapter 5—enzymes. Human Biochemistry, 95–129.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.