Fluid mosaic model
n., plural:
[fluːɪd məʊˈzeɪk mɒdl̩]
Definition: Model describing the structure of the plasma membrane as a dynamic and fluid arrangement of lipids, proteins, and carbohydrates
Table of Contents
Fluid Mosaic Model Definition
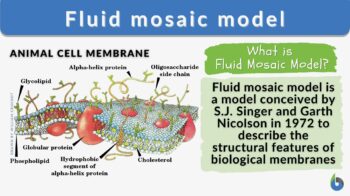
What is the fluid mosaic model? The fluid mosaic model is a three-dimensional representation of the structure and dynamics of the plasma membrane proposed by S.J. Singer and G.L. Nicolson in 1972. The fluid mosaic model describes the plasma membrane as a ‘fluid` and a ‘mosaic’ structure. According to this model, the plasma membrane is a phospholipid bilayer structure composed of fluid and a mosaic of diverse molecules, which is essential in understanding how the plasma membrane functions. The fluidity of such a biological membrane refers to its characteristic dynamicity. This means that the plasma membrane structure is dynamic rather than a rigid structure of lipids, proteins, and carbohydrates. The plasma membrane components have the ability to move within the plane of the membrane. There are other models but the fluid mosaic model is the widely accepted representation of the biological membranes to this day.
Let us learn more about the fluid mosaic model and find the answers to common questions like ‘What does the fluid mosaic model state? What is the cell membrane made of? How do the cell membrane components affect the general cell membrane functions?’ We can start with the fluid mosaic model definition in biology and what it states:
Biology definition:
A fluid mosaic model of the cell membrane (or plasma membrane) is a conceptual framework for its structure and behavior. This model states that the cell membrane is composed of a fluid lipid bilayer with proteins that are embedded within it. It emphasizes the fluid nature of the structure wherein the lipids and proteins are capable of lateral movement within the membrane as well as its mosaic nature as it contains a variety of proteins and lipids, as well as certain carbohydrates, that are distributed unevenly. This model acknowledges that the membrane is selectively permeable, allowing certain molecules to pass through while restricting the others. The selective permeability feature of the cell membrane is crucial to its role in cellular homeostasis by regulating the entry and exit of substances between the cell and its environment.
Development Of The Fluid Mosaic Model
Prior to the conception of the fluid mosaic model, the prevailing notion is that the cell membranes were static and rigid structures. This perception changed with the advancing studies on biological membranes using newer technologies, especially in microscopy techniques.
The development of the fluid mosaic model represented a significant paradigm shift in our understanding of cellular membranes. It transformed our perception of membranes from rigid barriers to dynamic entities, laying the foundation for further investigations into membrane biology and its implications in various cellular processes.
With the emergence of microscopes, the presence of cells as the fundamental unit of life has been recognized in the 1600s. Our understanding of the cells from then on expanded into identifying cellular parts, such as the cell membrane that was recognized in the early 1800s. Its permeability depending on conditions has also long been recognized.(Kalkan & Esrefoglu, 2020) (Hücre Membranının Keşfi: Tarihsel Bir Bakış, 2019) The chemical components of the cell membrane (i.e. lipids and proteins) were then identified in 1915.
How our understanding of the cell membrane was shaped? What are the key events that led to the development of the fluid mosaic model of the cell membranes? Let’s find out.
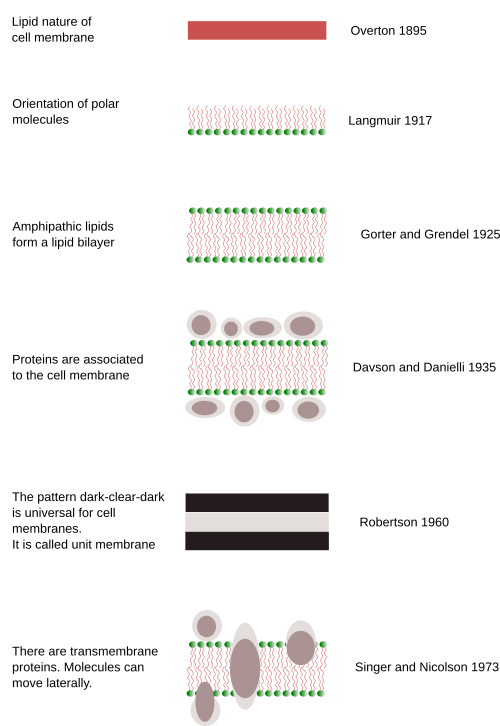
- » 1890s-Early 1900s: Charles Ernest Overton proposed the so-called Overton Biomembrane Model where he proposed biomembranes being made up of lipids based on his observation wherein lipid-soluble substances were transported across biomembranes. In 1904, Nathanson proposed a mosaic membrane theory, suggesting that the membrane contained mosaic domains to account for the different pathways for the entry of soluble or insoluble materials. (Hücre Membranının Keşfi: Tarihsel Bir Bakış, 2019)
- » 1920s: Evert Gorter and François Grendel proposed the lipid bilayer model in 1925, suggesting that the cell membrane consists of a double layer of lipids where the hydrophobic heads face outwards while the hydrophobic tails face inwards. Although incomplete, their model provided the basic framework of the cell membrane.
- » 1930s: The thought that the membrane was surrounded by proteins is suggested by Harvey and Kennet S. Cole in 1932. Then, in 1935, James Frederic Danielli and Hugh Davson Fricke proposed the sandwich membrane model (Davson-Danielli model or protein-protein model) in which a lipoid center is sandwiched between protein layers (i.e. protein-lipid-protein). Their work recognized the importance of proteins, particularly their role in various processes of biological membranes.
- » 1950s: Cell biologists were able to view cell membranes in significantly improved resolutions via electron microscopy. Membranes were seen as three layers. J. David Robertson came up with the unit membrane model where he theorized (1) that all cell membranes had a generalized tri-laminar structure (which corresponds to Danielli and Davson’s protein-lipid-protein arrangement of the cell membrane) and (2) that they perform a similar function.
- » 1960s: in 1964, Brady RO and Trams EG’s works laid important foundations for Singer and Nicolson’s model, such as the proteins entering the lipid matrix layer indicating that the membrane is fluid. (Kalkan & Esrefoglu, 2020)
- » 1970s: Seymour Jonathan Singer and Garth L. Nicolson proposed in 1972 the fluid mosaic model in their paper, “The Fluid Mosaic Model of the Structure of Cell Membranes” published in Science. Here, they introduced the fluidity of the cell membrane and its being composed of a mosaic of lipids and proteins.
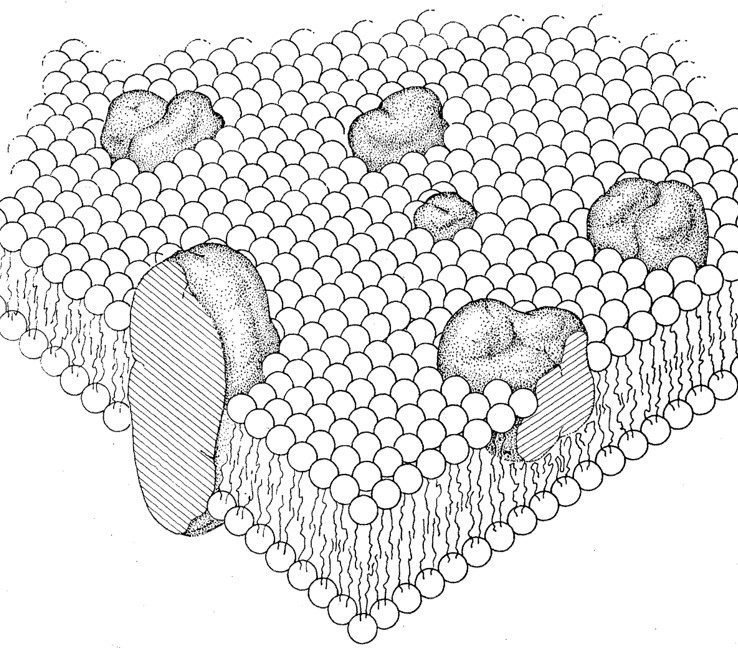
Over time, further research has been conducted that helped expand the understanding of the fluid mosaic model, refining and enhancing our understanding of biological membranes.
Various glycobiology researchers have contributed to the understanding of the glycocalyx and the role of carbohydrates in the cell membrane, which led to refinements of the fluid mosaic model by incorporating the spatial structure and role of carbohydrates, such as glycolipids and glycoproteins in the cell membrane.
Advances in microscopy in 1977 led to the visualization of the internal structure of the cell membrane, supporting the fluid mosaic model. Further advances in molecular biology and protein purification techniques in the ’80s and ’90s, in turn, aided in the characterization of integral membrane proteins, providing concrete evidence of the presence of proteins in the cell membrane. One of them is the joint work of Kai Simons and Elina Ikonen who published the article “Functional rafts in cell membranes” in Nature in 1997. They discussed the concept of “lipid rafts”, the cholesterol-rich microdomains in the cell membrane.
Through the work of scientists and researchers, the development of the fluid mosaic model has proven to be a significant milestone in cell biology as it paved the way to other important milestones, especially in the field of drug development and treatments.
Watch this vid about the fluid mosaic model:
Functions And Components Of Biological Membranes
Singer and Nicolson’s Fluid Mosaic Model provided several key insights into the features that help elucidate the components and functions of biological membranes. Some of them are as follows:
- Fluidity: Singer and Nicolson’s model highlighted that the cell membrane is a dynamic and fluid structure. Being composed of a lipid bilayer, the cell membrane behaves like a liquid in which the individual lipid molecules can move laterally within the membrane plane. Fluidity is an important feature of biological membranes because it enables the following membrane functions:
- Selective permeability. Certain molecules can diffuse unaided through the membrane easily (while preventing most substances to pass through).
- Membrane protein functions. The proteins can also allow movement within the lipid bilayer. With proteins moving laterally, it allows biological membranes to carry out processes, such as protein-protein interactions and signal transductions.
- Vesicle Formation and Fusion. Due to membrane flexibility, it allows the membrane to undergo curvature and thus, create vesicles for the transport of substances within and outside the cell.
- Cellular homeostasis. Because the membrane has the ability to alter its shape and remodel, it can therefore repair itself by fusing to maintain its structural integrity, which is essential for cellular homeostasis.
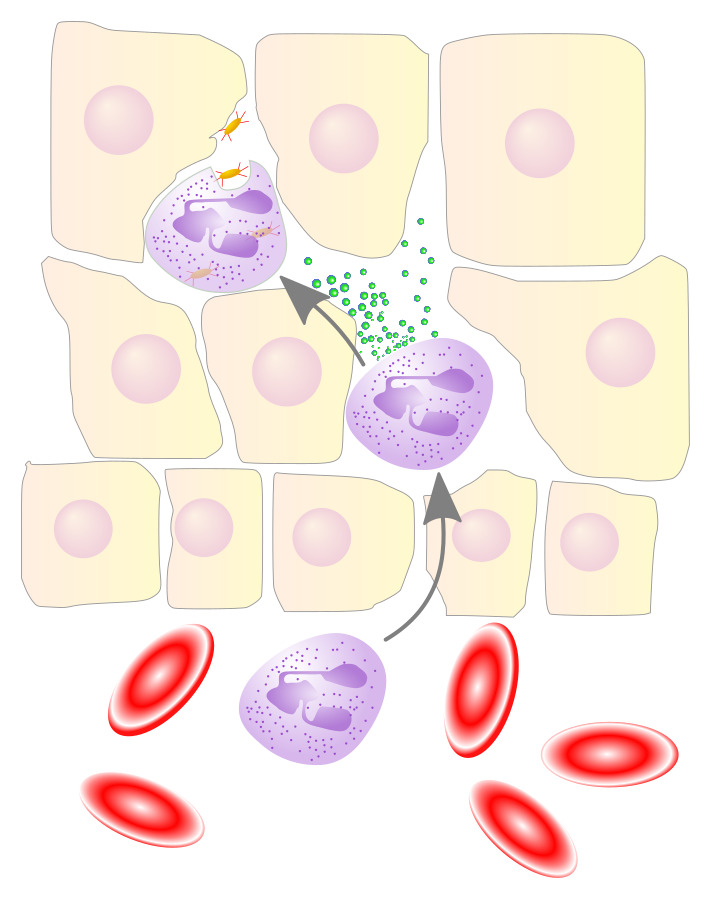
- Cell migration. Membrane fluidity enables certain cells to migrate or move, such as leukocytes passing through blood vessel walls into injured tissues.

Figure 3: Neutrophile migration. Image Credit: Xu et al., 2022
- Mosaic Arrangement: Singer and Nicolson’s model likened the cell membrane to a mosaic, i.e., made up of various components, such as lipids, proteins, and carbohydrates. This contributes to the cell membrane asymmetry. The differential distribution of lipids and proteins between the two leaflets of the lipid bilayer of the biological membranes renders them distinct properties and functions.
- Selective permeability. While some small, non-polar molecules (e.g., oxygen and carbon dioxide) can diffuse freely through the lipid bilayer due to the membrane’s flexibility and fluidity, larger molecules and ions cannot. However, they can still pass through albeit not as easily. Their transport will be regulated via specialized transport proteins before they can be allowed to cross the membrane.
- Efficient cell signaling. The asymmetry in the cell membrane enables one side of the membrane to be concentrated with signaling molecules. This is crucial in certain events, such as in the detection of external signals and leading to the initiation of the appropriate response.
- Specialized function. Because membranes would vary in spatial arrangements and compositions, the function of the cell would also therefore vary. Their cell membrane will be able to carry out special functions, depending on the cellular needs and environmental changes.
- Improved communication. For instance, the cell will present signals on the outer surface of its cell membrane to trigger target cells to act on it.

Figure 4: A tumor cell presenting antigen on the cell membrane surface. T cell is able to recognize it via its antigen and therefore will be induced to act upon it to eliminate it. Image credit: BiTE_antibody_01.svg: Kuebi, CC BY-SA 3.0 Unported. - Efficient trafficking. The asymmetrical distribution of proteins in a biological membrane is crucial to various biological processes, such as endocytosis. For instance, clathrin-coated vesicles formed during receptor-mediated endocytosis exhibit membrane asymmetry. The cytoplasmic face of the vesicle membrane contains proteins such as clathrin and adaptins that mediate the vesicle formation. The luminal face (i.e. facing the extracellular matrix or space), in turn, displays receptor proteins that have undergone internalization. Not only is this crucial in cell membranes. Asymmetry is also important in the plasma membranes of the mitochondria. In particular, the inner mitochondrial membrane (which is the site of the electron transport chain of cellular respiration) is also the site of ATP synthases, which pump H+ ions into the mitochondrial lumen, for ATP production.
- Protein-lipid interactions: The model recognizes the lipid-protein interactions, which are crucial in maintaining structural integrity. The lipid bi-layer interacting with the proteins embedded in it provides a stable barrier that is crucial to a cell.
- Firstly, such a structural and physiological organization enables the cell membrane to efficiently serve as a protective covering against pathogens and harmful substances.
- Secondly, it enables compartmentalization. Plasma membranes create distinct compartments within cells, enabling efficient organization and specialization of cellular processes.
How the cell membranes (plasma membranes) are able to possess these characteristics is due to the membrane structures that made them. Here are the fundamental biological membrane structures: The presence of unsaturated fatty acids introduces kinks in the hydrocarbon chains, which contributes to the membrane’s fluidity.
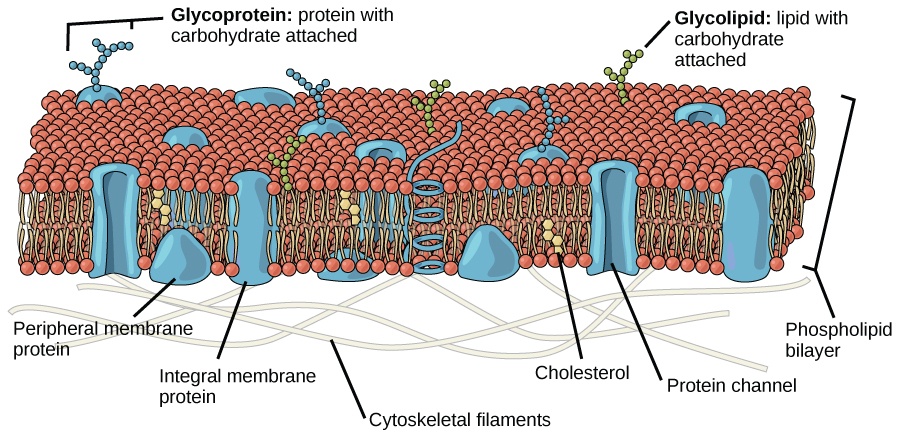
Phospholipids
In Figure 2, the model shows a membrane bilayer of phospholipid molecules that make up the bulk of the structure. In Figure 5 below, take note of the phosphoglyceride (the primary type of membrane phospholipids). It is made up of two parts: the head, which is made up of phosphate and glycerol molecules, and the tail, which is made up of saturated and unsaturated fatty acids. Anther membrane phospholipids are sphingolipids (have a sphingosine backbone rather than a glycerol backbone).
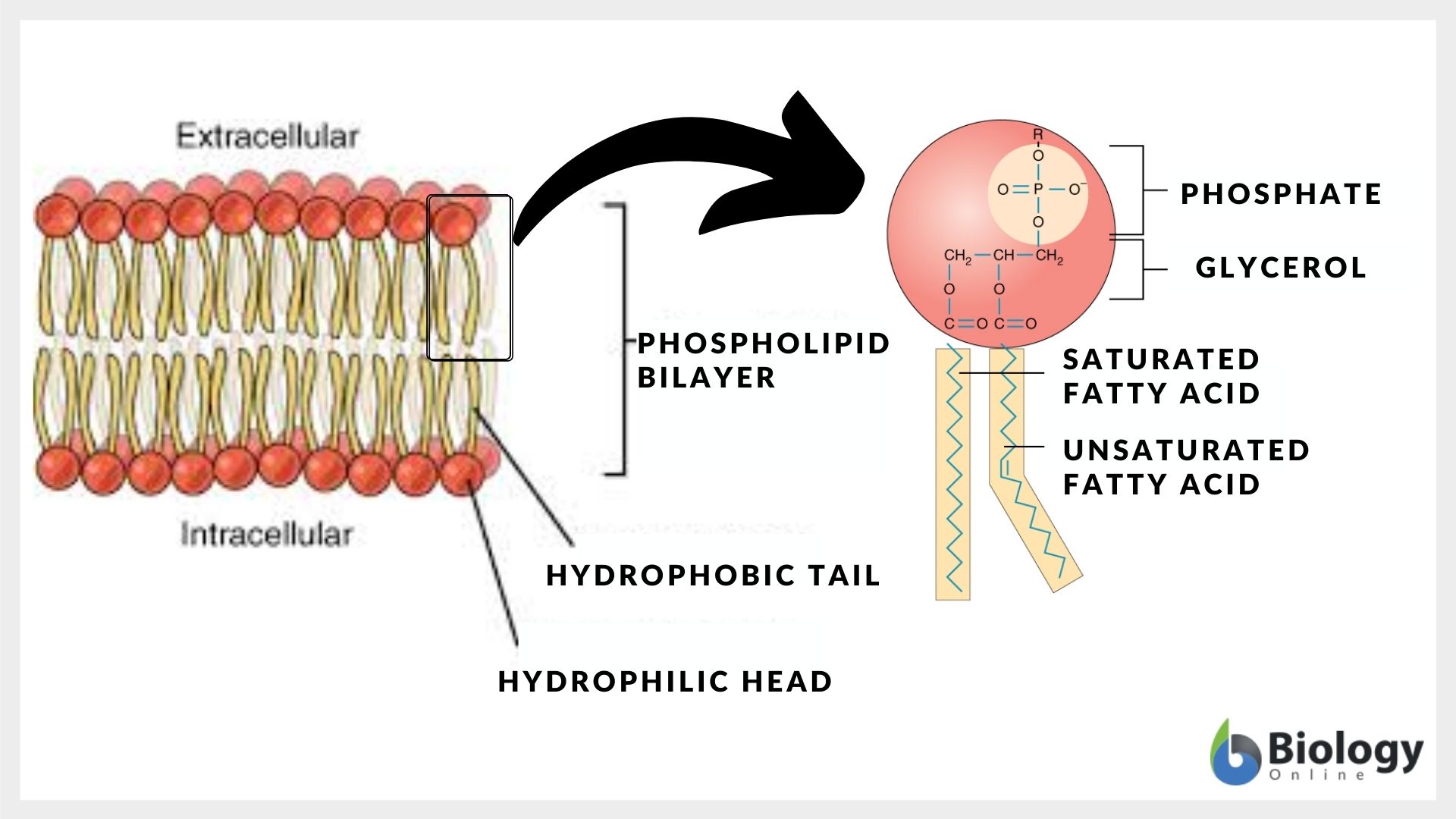
The phospholipids are amphipathic molecules. This means they have both water-loving (hydrophilic) and water-fearing (hydrophobic) regions. The hydrophobic core forms from the hydrophobic interactions of the tails resulting in a bilipid layer orientation wherein the hydrophilic heads face toward the aqueous environment.
These phospholipids are capable of moving laterally within the plane of the membrane. They can also move transversely (referred to as flip-flop) although they do so infrequently and with the aid of enzymes called flippases.
The phospholipids provide a means by which the membrane proteins can be inserted or embedded, creating membrane domains (specialized regions within the membrane composed of lipids and protein complexes). They are also capable of interacting on the cell membrane surface with other molecules, such as carbohydrates and other proteins for cell signaling and communication.
Proteins
Singer and Nicoson highlighted two types of proteins in cell membranes:
- Peripheral membrane proteins: peripheral proteins are proteins present or associated with the membrane surface (i.e., cell surface facing the inside or the outside of the cell), hence, the name “peripheral”). See Figure 1.
- Integral membrane proteins: integral proteins are cell membrane proteins that notably contribute to the structure of the membrane. Their hydrophobic region interacts with the hydrophobic core of the lipid bilayer, thus, are able to anchor themselves in place. Because they are fairly embedded they are sometimes described or referred to as intrinsic proteins. They span the lipid bilayer partially or completely. For instance, transmembrane proteins span the entire width of the lipid bilayer, extending into both the cytoplasm and the extracellular fluid. See Figure 1.
Carbohydrates
Figure 1 shows carbohydrates protruding on the outer cell surfaces. Carbohydrates when attached to membrane lipids form glycolipids. Other carbohydrates attach to proteins, forming glycoproteins. The carbohydrate chains extend outward into the extracellular space. A layer of glycolipids or glycoproteins forms glycocalyx.
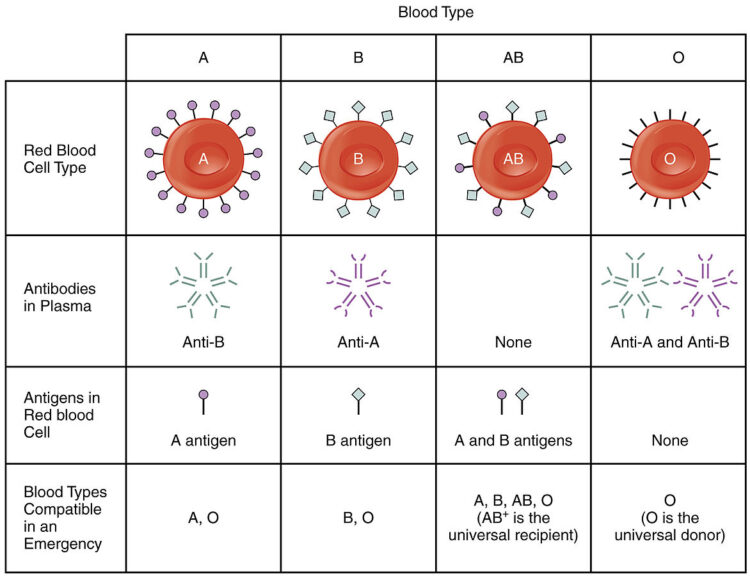
An example of glycoproteins on the membrane surface is the major histocompatibility complex (MHC) protein, which is a group of glycoproteins in the cell membranes of nucleated cells in humans and other vertebrates. Another is the glycosyltransferases, which when functional, can add specific sugars to H antigen to form A antigen and/or B antigen. Together, they are transported to the cell surface where the glycosyltransferases eventually become part of the cell membrane akin to the integral membrane proteins and display the antigen sugars on the surface of the red blood cells.
NOTE IT!
Did you know that the ABO blood type test is based on certain carbohydrate antigens?
The carbohydrate antigens of red blood cell membranes are used as a basis for blood typing tests. In particular, the test is based on the presence or absence of the ABO blood group system, which is a system of specific carbohydrate molecules that act as antigens on the surface of red blood cells. These antigens are A, B, and H antigens. Here is a chart to explain how the different blood types are determined based on the antigens present in the RBCs.
Cholesterol
Although the seminal paper of Singer and Nicolson did not specifically mention cholesterol, subsequent studies have eventually recognized cholesterol as part of the lipid composition of the cell membrane.
(While the traditional view of cholesterol is primarily associated with animal cell membranes only, there are some interesting discoveries of recent implicating that cholesterol molecules may occur as well in certain types of cells of other groups of living things, such as bacteria and plants, which have only phytosterols or cholesterol-like compounds. If so, cholesterol would still rather be more common in animal cells.)
Remember that animal cells lack a cell wall, and therefore, the presence of cholesterol would help in providing structural support. Cholesterol is essential in maintaining the structural fluidity, integrity, and functionality of cell membranes.
How does cholesterol affect membrane fluidity? What is the function of cholesterol in the cell membrane? Cholesterol is an amphipathic lipid molecule embedded within the phospholipid core. It acts as a “fluidity buffer” by reducing the fluidity of the membrane at high temperatures and increasing it at low temperatures.
At high temperatures, cholesterol restrains the movement of phospholipids, reducing their mobility and preventing excessive fluidity. At low temperatures, cholesterol prevents the close packing of phospholipids, increasing their mobility and maintaining fluidity.
Apart from fluidity, cholesterol also plays a role in the formation of lipid rafts serving as platforms for various cellular processes, including signal transduction and membrane trafficking.
Other Models For Membrane Structure
There have been other models proposed to describe membrane structure. Nevertheless, the fluid mosaic model remains widely accepted.
For details, see Development Of The Fluid Mosaic Model.
Apart from the ones mentioned above, here are other models that have provided additional insights and perspectives on the organization and behavior of cell membranes:
- Lipid Raft Model: proposes that the cell membrane is organized into distinct microdomains called lipid rafts, enriched in specific lipids and associated proteins.
- Protein-Induced Bilayer Deformation Model: suggests that proteins act as scaffolds that can induce local deformations in the lipid bilayer structure, leading to changes in membrane curvature and organization.
Take the Fluid Mosaic Model – Biology Quiz!
Further Reading
References
- Singer, S.J., & Nicolson, G.L. (1972). The fluid mosaic model of the structure of cell membranes. Science, 175(23), 720-731.
- Simons, K., & Ikonen, E. (1997). Functional rafts in cell membranes. Nature, 387(6633), 569-572.
- McMahon, H.T., & Gallop, J.L. (2005). Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature, 438(7068), 590-596.
- Molnar, C., & Gair, J. (2015, May 14). 3.4 The Cell Membrane. Opentextbc.ca; BCcampus. https://opentextbc.ca/biology/chapter/3-4-the-cell-membrane/
- Kübra Tuğçe Kalkan, & Mukaddes Esrefoglu. (2020). The Cell Membrane: A Historical Narration. 8(1), 81–88. https://doi.org/10.14235/bas.galenos.2019.3131
- Hücre Membranının Keşfi: Tarihsel Bir Bakış. (2019). Bezmialemscience.org. https://www.bezmialemscience.org/archives/archive-detail/article-preview/the-cell-membrane-a-historical-narration/34643#:~:text=In%201904%20Nathanson%20put%20forward,presipitation%20membrane%20(artificial%20membrane).
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.