Immunoglobulin
n., plural: immunoglobulins
/ˌɪmjuːnəˈɡloʊbjuːlɪn/
Definition: Antibody proteins produced by B cells.
Table of Contents
Immunoglobulin Definition
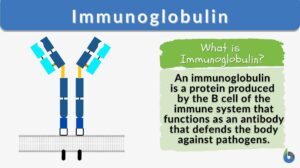
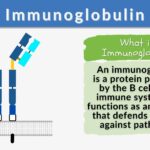
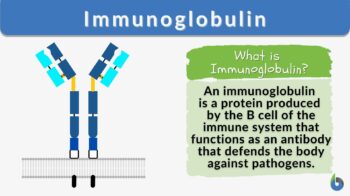
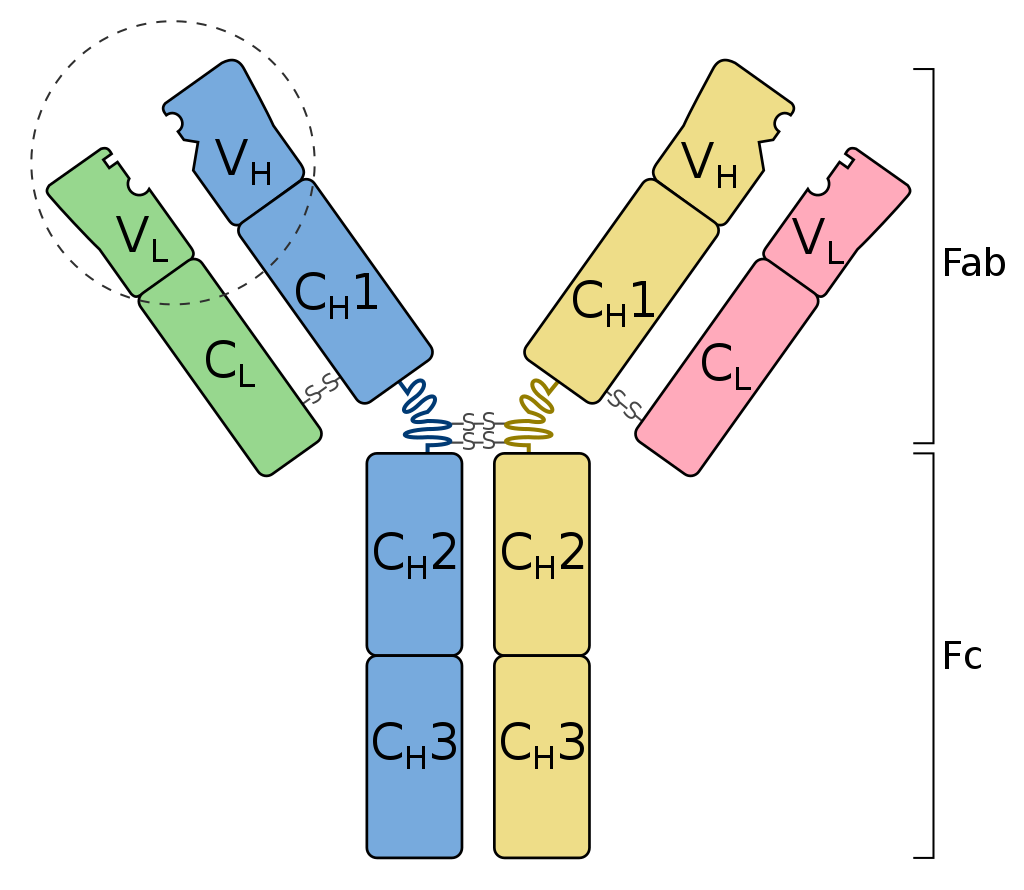
An immunoglobulin is a globulin molecule produced by the immune cells, for the body’s defense against antigens or foreign substances, by recognizing and binding with them. In essence, there are three regions:(1) Fab fragment, the antigen-binding region, which includes the antigen-binding site, (2) Fc fragment, the crystallizable region, which consists of the remaining constant sequence domains of the heavy chains, cell-binding site, and complement-binding sites, and (3) Transmembrane domain and a cytoplasmic tail to bind on the surface of the plasma cell.
The fundamental structure (monomer) of an immunoglobulin is a Y-shaped unit consisting of two heavy chains and two light chains. In each of these chains, there are variable and constant regions. The apical portion of the variable regions serves as an antigen-binding site.
Etymology: The term “immunoglobulin” derives from “immuno-” (related to immunity or the immune system) and “globulin” (a type of protein).
Synonym: antibody
Compare: antigen
Abbreviation: IG
Immunoglobulin vs. antibody
Immunoglobulin and antibody are two terms that are used interchangeably as both are generally defined as glycoprotein molecules that are structurally the same and with corresponding regions. Their primary role in the immune system is for the recognition of antigens (foreign materials) inside the body.
However, in some literature, the two terms are used differently. In particular, immunoglobulins are those found on the surface of the B cell via the transmembrane domain that enables them to attach to the plasma membrane of the B cell via the B cell receptors. Antibodies, in contrast, are those that do not have a transmembrane domain and thus float in the circulation.
(Lakna Panawala, 2017) There are other references, though, that see antibodies as immunoglobulins, and in this case, are classified as either membrane-bound or secreted form (freely floating) immunoglobulins. (Bansal, 2022) In free forms, such soluble antibodies are found circulating in blood plasma or secreted in mucus and other bodily secretions.

Common Features of Immunoglobulins
Here are the common features shared by immunoglobulins:
- Globulins: Immunoglobulins, as their name implies, are globulins (a major group of plasma proteins along with other protein groups in blood plasma and other body fluids, such as fibrinogen and albumin).
- Y-Shaped Structure: Immunoglobuins have a distinctive Y shape where each arm consists of a heavy chain (longer polypeptide chain) and a light chain (shorter polypeptide chain).
- Variable and constant regions: Each chain has two regions: variable region and constant region. The variable regions are responsible for antigen recognition and have unique sequences, whereas the constant regions determine the immunoglobulin classes (e.g., IgG, IgG, IgA, IgM) and effector functions.
- Fab (Fragment antigen-binding) and Fc (Fragment crystallizable) regions:
- Fab region is the antigen-bindig region responsible for antigen recognition and binding ability. It includes the variable region and portions of the constant regions of heavy and light chains. Each immunoglobulin or antibody has unique Fab regions that enable it to recognize and bind to a specific antigen with high affinity.
- Fc region is the constant region responsible for many of the effector functions, such as opsonization, complement activation, and interaction with immune cells. It is entirely composed of the constant regions of the heavy chais of the antibody. It can bind with the Fc receptors on the cell surface of the effector white blood cells, such as macrophages, neutrophils, natural killer cells, and B cells.
- Disulfide bonds: This chemical bond links the heavy chain with the light chain and the Fab and Fc regions. This type of bond helps stabilize the structure of the immunoglobin, ensuring the proper folding of the Y-shaped molecule.
- Antigen-binding sites: Identical antigen-binding sites are found in the upper portion of the arms. They are responsible for the recognition and binding with specific antigens.
- Origin: Immunoglobulins are primarily produced B cells, which in turn, originated from the bone marrow. Some B cells, when activated, undergo lymphocyte differentiation and become and specialized B cells, such as plasma cells and memory B cells, that produce and secrete specific antibodies.
- Primary function: Immunoglobulins are capable of recognizing antigens and other immune-related functions.
Types Of Immunoglobulins
In placental mammals (including humans), there are five classes (or antibody isotypes) of immunoglobulins based on biological activity:
- IgG (Immunoglobulin G): IgG antibodies are the most abundant in the bloodstream and provide long-lasting protection against infections. They can cross the placenta to confer passive immunity to newborns.
- IgA (Immunoglobulin A): IgA antibodies are prevalent in mucous membranes, saliva, tears, and breast milk, serving as the body’s first line of defense against pathogens at these entry points.
- IgM (Immunoglobulin M): IgM antibodies are the first antibodies produced during an immune response and are often found on the surface of B cells. They play a crucial role in initiating the immune response.
- IgD (Immunoglobulin D): IgD antibodies are primarily found on the surface of B cells, where they assist in the activation of these cells. Their exact role is still being explored.
- IgE (Immunoglobulin E): IgE antibodies are involved in allergic reactions and defense against parasitic infections. They are found in small amounts in the bloodstream.
Antibodies and Their Function (by FreeMedEducation):
We’ve mentioned that the immunoglobulins are so named because they are globulins with immune function. They can therefore be identified based on their electrophoretic behavior (migration pattern in electrophoresis) and immunoglobulin structure:
- immunoglobulin A (IgA) – a dimer with α (alpha) heavy chain
- immunoglobulin D (IgD) – a monomer with δ (delta) heavy chain
- immunoglobulin E (IgE) – a monomer with ε (epsilon) heavy chain
- immunoglobulin G (IgG) – a monomer with γ (gamma) heavy chain; usually referred to as “gamma globulins”
- immunoglobulin M (IgM) – a pentamer with μ (mu) heavy chain
Functions Of Immunoglobulins
Immunoglobulins perform several critical functions:
- Neutralization: Antibodies bind to pathogens, preventing them from infecting host cells.
- Opsonization: Antibodies mark pathogens for destruction by immune cells.
- Complement activation: Antibodies can trigger the complement system, enhancing the immune response.
NOTE IT!
Immunoglobulins are essential components of the immune system, and provide defense against a wide range of pathogens and diseases. Understanding the roles of specific antibodies in the immune response is fundamental in both diagnostics and the development of immunoglobulin test, blood sample test measures, and therapeutic strategies, such as intravenous immunoglobulin therapy, which uses purified antibodies to treat immunodeficiency disorders, autoimmune diseases, and other conditions related to malfunctioning immunoglobulin levels, such as immunoglobulin deficiency (too few immunoglobulins than the normal level).
References
- Lakna Panawala. (2017, October 20). Difference Between Immunoglobulin and Antibody. ResearchGate; unknown. https://www.researchgate.net/publication/320583004_Difference_Between_Immunoglobulin_and_Antibody#:~:text=Immunoglobulins%20are%20attached%20to%20the,not%20have%20a%20transmembrane%20domain.
- Bansal, R. (2022, May 25). Membrane-bound Immunoglobulins and B-cell Receptor (Part 11- Antibody Basics). Medium; Biotechnology by TSB. https://medium.com/biotechnology-by-tsb/membrane-bound-immunoglobulins-and-b-cell-receptor-part-11-antibody-basics-c3aabc510562
- Janeway’s Immunobiology by Kenneth Murphy
- Molecular Biology of the Cell by Bruce Alberts
- Abbas, A. K., et al. (2020). Cellular and Molecular Immunology. Saunders.
- Alberts, B., et al. (2017). Essential Cell Biology. Garland Science.
- Male, D., et al. (2016). Immunology. Elsevier.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.