Phagocytosis
n., plural: phagocytoses
[ˌfeɪ.ɡəʊ.saɪˈtəʊ.sɪs]
Definition: Cellular engulfing and digestion of foreign particles or pathogens
Table of Contents
Phagocytosis Definition
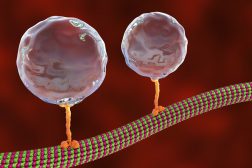
Phagocytosis is a basic physiological cellular process wherein a cell ingests a solid particle having a diameter >0.5 µm. In unicellular organisms, it is a feeding mechanism to acquire nutrition while in multicellular organisms, it is used as a defense mechanism to remove pathogens from the body. The process of phagocytosis involves the identification of the material/particle (having a diameter>0.5 µm) followed by its ingestion into a vesicle formed by extending the plasma membrane and is known as phagosome (Figure 1).

Phagocytosis is a type of endocytosis phenomenon. However, it differs from the endocytosis. Endocytosis is a highly specific phenomenon wherein the cell recognizes the particle/microorganism to be engulfed with the help of a cell receptor. Also, physical contact between the target particle/microbe and the phagocyte cell can trigger phagocytosis.
In unicellular organisms (like amoeba, sponges), phagocytosis is a process for obtaining food and nutrition. In multicellular organisms, there are specialized cells (white blood cells, monocytes, neutrophils, etc) that carry out phagocytosis and are referred to as phagocytes.
Phagocytes use phagocytosis to eliminate foreign substances, pathogenic microbes, apoptotic cells, and cell debris, thus helping in maintaining homeostasis. The process of phagocytosis is one of the fundamental processes carried out by the innate immune system in the human body. Phagocytosis is one of the initial steps taken by the immune system when it encounters a pathogen/infectious substance. Phagocytosis is one of the immune response-initiating the branch of the adaptive immune system that is mediated by T cell receptors and T cells.
Watch this vid about phagocytosis by a human neutrophil:
Biology definition:
Phagocytosis is a basic physiological cellular phenomenon wherein the cell eats or engulfs particles, microorganisms, or foreign substances having a diameter of more than 0.5 µm. Phagocytosis is the mechanism used by many protists (e.g. amoeba) to acquire nutrients. In humans and other multicellular animals, phagocytosis is an important defense mechanism against infection by microorganisms (e.g. bacteria) and the process of removing cell debris (e.g. dead tissue cells) and other foreign bodies. The process of engulfing and ingestion of particles by the cell or a phagocyte (e.g. macrophage) forms a phagosome (or food vacuole), which in turn fuses with a lysosome and becomes a phagolysosome where the engulfed material is eventually digested or degraded and either released extracellularly (via exocytosis) or intracellularly (via vesicle transport) for further processing.
Main steps of a macrophage ingesting a pathogen:
a. Ingestion through phagocytosis, a phagosome is formed
b. The fusion of lysosomes with the phagosome creates a phagolysosome; the pathogen is broken down by enzymes
c. Waste material is expelled or assimilated.
Etymology: Phagocytosis = phago (Greek word) + cyte (Greek word), “devouring” or “to eat cell”. Hence, the literal meaning of Phagocytosis is cell eating.
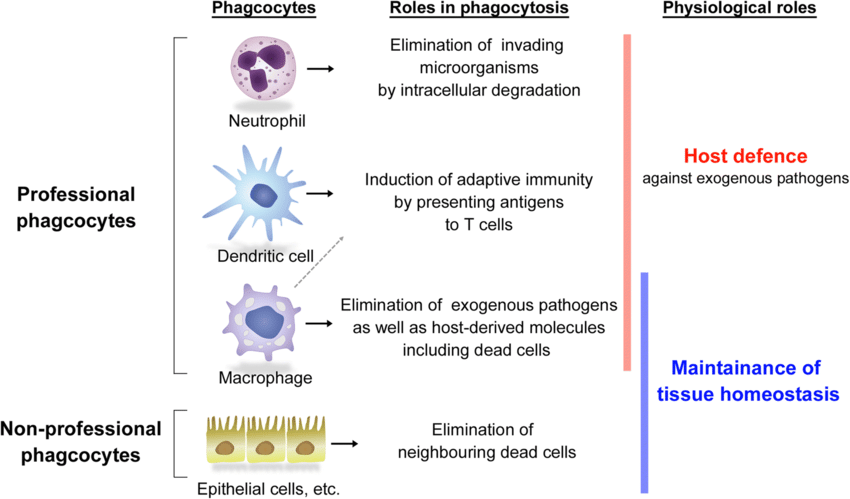
Types of Phagocytes
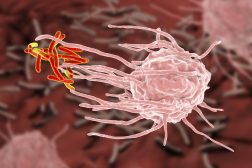
Even though most of the cells are capable of carrying out the process of phagocytosis, certain cells are specialized in the process of phagocytosis and carry out the process of phagocytosis with a high degree of efficiency. Such specialized cells are referred to as professional phagocytic cells. (Figure 2)
Some of the professional phagocyte cells include:
Only specialized cells, i.e., professional phagocytes, are highly specific and can identify the pathogen/ foreign particles with high specificity and efficiency. These cells help to eliminate the pathogen and also present antigens to the lymphocytes to activate the adaptive immune response of the body.
Certain cells can also undergo or perform the phagocytosis process, however, the efficiency of the process is low. These cells are referred to as Non-professional phagocytic cells. Some of the non-professional phagocytic cell types include:
Non-phagocytic cells carry out the process of removing apoptotic bodies, however, these cells can not carry out the ingestion of microorganisms. Hence, these cells are helpful in maintaining homeostasis.
Phagocytic Receptors
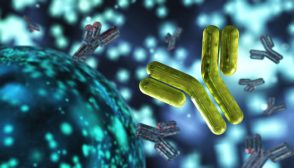
To initiate phagocytosis, the cells must first recognize or identify the substance to be phagocytosed. Phagocytotic cells carry out the identification process with the aid of receptor-mediated phagocytosis (Figure 3). The recognition of pathogen/foreign particles is carried out by surface receptors.
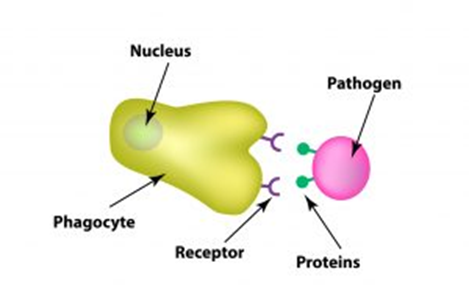
These discrete receptors are located on the plasma membrane of the phagocytes and are of the following types:
Opsonic receptors
These receptors recognize the host cell-derived opsonins that get attached to the foreign particle and thus making them a target for phagocytosis. Opsonic receptors bind bacteria and other pathogens with Immunoglobulin (Ig) G antibodies of the immune system thus enabling other cells of the immune to identify the potential threat to the body.
Opsonic receptors include fibronectin, antibodies, mannose-binding lectin, milk fat globulin (lactadherin), and complement receptors. Of these, in complement-mediated phagocytosis, complement receptors and Fc receptors (FcR) are the most vital receptors.
- The complement receptors get bind to the iC3b which is found on the particle/cell surface and thereby activating the complement system signaling. A complement system is a group of proteins that tag the pathogen or the bacteria so that immune cells can identify the potential threat to the body. Opsonic receptors foreign particles are labeled by the phagocyte with the help of opsonins in order to facilitate and activate phagocytosis.
- Fc receptors bind to the Fc portion of Ig G and Ig A (Immunoglobulin) in fcγ receptor-mediated phagocytosis. Fcγ crosslinks with IgG and activates Src family kinases (SFK), which activates the internalization process in phagocytosis through phosphorylate tyrosine residues. SFK is also involved in the process that regulates Rac activation and contractile proteins such as myosin during cell adhesion.
Non-opsonic receptors
The other phagocytic receptors are non-opsonic receptors that directly identify the molecules present on the surface of the pathogen or target. These include:
- C-type lectins, like, Dectin-2, mannose receptor, Mincle, or DNGR-1
- Lectin-like recognition molecules, such as CD169 and CD33
- Scavenger receptors: The majority of bacteria produce a protein substance around them (also known as extracellular matrix), which is used by phagocytes to identify them as targets. Human cells do not produce this extracellular matrix protein substance and hence provide specificity to the phagocytes to identify them.
- Dectin-1- a receptor for fungal beta-glucan
- SR-A or CD36
- Other receptors like Toll-like receptors or TLRs also help in the identification of bacteria/foreign substances, however, TLRs do not function as a receptor for phagocytosis. TLRs in combination with other non-opsonic receptors induce phagocytosis.
Phagosome formation and its fate
Once the identification of the target cell/substance is complete, a signaling cascade is initiated in the phagocyte cell that initiates the lipid remodeling in the cell membrane. Also, membrane-associated cortical cytoskeleton, i.e., actin cytoskeleton, of the phagocyte is induced for extending the cell membrane around the target cell i.e., phagosome formation. Actin filaments are one of the contractile proteins present in the microtubule cytoskeleton of the cell. This signaling pathway also activates myosin ii which helps actin cytoskeleton to form phagosome.
Once the early phagosome internalizes the target cell/particle/substance, the vesicles from Golgi bodies and endoplasmic reticulum fuse with the early phagosome to initiate the formation of the intermediate phagosome. Once an intermediate phagosome is formed with endocytic vesicles fusing with it, remodeling of the phagosome membrane and eventual acidification of the phagosome. Eventually, this intermediate phagosome fuses with the lysosomes to get converted to a phagolysosome through the process of phagolysosome maturation. Phagolysosome is a microbicidal vacuole wherein the highly acidified environment activates the enzymes that digest and inactivate the foreign particle and microbial pathogens.
Function Of Phagocytosis
In unicellular organisms, phagocytosis is the process of acquiring food and nutrition for life processes. In multicellular organisms, it is an essential step in the immune system of the body to eliminate the pathogens/infectious agents/foreign substances that attack the human body. Thus, phagocytosis helps in protecting the body by eliminating external harmful substances/microbes. Also, phagocyte cells engulf or eat the non-functional cells, thus, acting as an aid to maintain the homeostasis of the body.
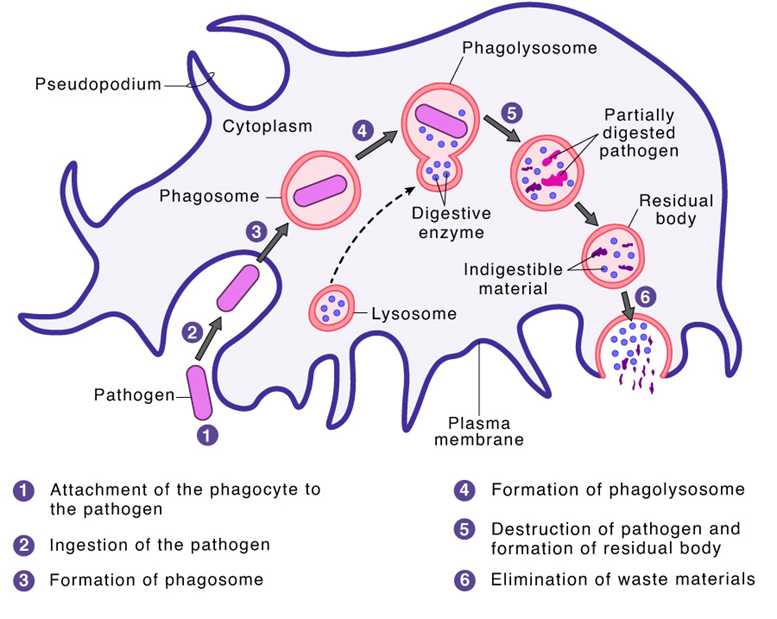
Steps Of Phagocytosis
Here are the general steps of phagocytosis. (Figure 4)
Step 1: Receptor binding and activation of phagocytes
The first phase of phagocytosis is receptor binding and activation of phagocytes. The activation of resting phagocytes is mediated by inflammatory mediators signaling pathways that involve signaling molecules like cytokines, prostaglandins, and bacterial products like lipopolysaccharides (LPS), capsules, teichoic acid, peptidoglycan, etc. These inflammatory mediators induce the production of glycoproteins on the surface of phagocytes that enable them to bind to capillary walls and eventually squeeze them out of capillaries and reach the target site or infection site.
Also, phagocytes produce endocytic pattern-recognition receptors (cell surface receptors) that identify and attach to the pathogen-associated molecular patterns or PAMPs (which include LPS, teichoic acid, mannose-rich glycans, and peptidoglycans) leading to the attachment or binding of the pathogen to the phagocyte. Activating receptors to identify the pathogen /foreign particle is one of the initial steps of phagocytosis.
Also, inside the phagocytic cell, there is an increase in the metabolic activity to generate the energy or ATP, lysozyme, etc.
Step 2: Chemotaxis of phagocytes
Chemotaxis of phagocytes is the second step in the process of phagocytosis. Herein, the phagocytes move towards the high-concentration region containing the same kind of attractants which may be, LPS, teichoic acid, mannose-rich glycans, and peptidoglycans from the bacteria, phospholipids from the injured host cells, inflammatory cytokines like Interleukin 8 and kinins.
Bacterial cells like Mycobacterium tuberculosis, Bordetella pertussis, and Neisseria gonorrhoeae and viruses like, influenza A virus has the ability to block this chemotaxis of the phagocytes.
Step 3: Attachment of phagocytes to the pathogen or cell
The attachment of phagocytes to the pathogen or cell is the third step in the process of phagocytosis. For phagocytosis to occur, the attachment of the phagocyte with the pathogen is pertinent. The attachment could be either:
- Unenhanced attachment of bacteria to phagocyte– This type of attachment of bacteria to phagocyte is mediated by PAMPs components like lipopolysaccharides, glycoproteins, peptidoglycans, teichoic acid, glucans, and mannans (released by the pathogen), which are recognized by the glycoprotein molecules present on the surface of the phagocytes and are referred to as molecules known as pattern-recognition receptors.
- Enhanced attachment of bacteria to phagocyte– This type of attachment is mediated by IgG antibodies that function to bind the pathogen to the phagocytic cell through pathogenic proteins and polysaccharides. In certain cases, multivalent antigen-antibody complexes are also involved in the binding with the antigen or pathogen. The Fab portion or the tip of the IgG molecule binds to the antigen having a complementary shape while the Fc potion or the tail portion binds to the pathogenic Fc receptors. Upon the activation of the complement system in the body, there is a production of C3b, a protein. This C3b gets attached to the pathogen or bacterial surface proteins on one end while the other end is attached to the C3b receptors on phagocytes. C3b and IgG are referred to as opsonins and the process is referred to as opsonization.
Integrins (transmembrane glycoproteins) in the phagocytes are the receptors that promote cellular adhesion. Aggregation of FcγR activates Integrins. Integrin activation facilitates the binding of the cell with the extracellular matrix. The activated integrin which gets bind to the particle forms a diffusion barrier for larger molecules like transmembrane phosphatase CD45.
Certain bacteria (like Streptococcus pneumonia, Neisseria meningitidis, Neisseria gonorrhoeae, Streptococcus pyogenes) have developed the capability to escape the attachment of bacteria to the phagocyte thus escaping the immune system of the body as they inhibit phagocytosis.
Step 4: Ingestion of the pathogen by phagocyte
Ingestion of the pathogen by phagocyte is the next step in the process of phagocytosis. The attachment of phagocytes with the pathogen induces a cascade of reactions that promotes actin polymerization of the actin-binding proteins followed by depolymerization of the actin filaments which results in protrusion of the cell membrane to form phagosome. Guanine nucleotide exchange factors are involved in the activation of the actin cytoskeleton.
For the complete formation of the phagosome, the last step involves the removal of F-actin from the base of the phagocytic cup, prior to the membrane protrusions fusing, to join and seal both ends to form the nascent phagosome. The phagosome cup or vesicle formation is completed by facilitating actin disassembly.
The depolymerization occurs through f actin debranching proteins like cofilin. Within the vesicular phagosome, the pathogen or the microbe is ingested. Actin cytoskeleton is used in FcγR-mediated phagocytosis while actin and microtubule cytoskeletons are engaged in CR-mediated phagocytosis.
Step 5: Digestion of pathogens
Digestion of pathogens is the next step in the process of phagocytosis. Once, the pathogen is ingested and is present inside the phagosome, H+ (hydrogen ions) are pumped inside the phagosome by the electron pumps. This results in a lowering of the pH of the phagosome or acidification of the phagosome (pH~3.5 to 4.0). Under this situation, when the lysosomes fuse with the phagosome the resultant pH activates the acid hydrolases.
Acid hydrolases break down the protein and other cellular components of the pathogen. Simultaneously, cathelicidin, bacterial permeability inducing protein (BPI), defensins, early endosome antigen 1, lipases, proteases, and peptides and enzymes are also released due to low pH and these enzymes digest the cellular component of the pathogen. Lysosomal-associated membrane protein is the most abundant component of lysosome that fuses with the phagosome.
Certain bacteria like Mycobacterium tuberculosis, Listeria monocytogenes, Salmonella, and Yersinia can block phagocytic ingestion.
Cancer cells block phagocytic ingestion by signaling regulatory protein α expression, which conveys the phagocyte to evade its ingestion. Extracellular signal-regulated kinases, a type of protein kinase, are also involved in the evasion of phagocytosis of cancer cells.
Step 6: Removal of digested material from the phagocyte
Removal of digested material from the phagocyte is the last step in the process of phagocytosis. With the help of the process of exocytosis, the waste, digested material, which can not be further reused by the cell, is eliminated from the phagocyte. Exocytosis is a reverse process of phagocytosis wherein the material to be eliminated like dead tissue cells is enclosed within a vesicle and moves along with it to reach and fuse with the cellular membrane and eventually, released into the external environment.
Examples Of Phagocytosis
Amongst the unicellular organisms, phagocytosis is seen in amoeba. Amoeba, when comes in contact with the food material, extends pseudopods around the food and engulfs the food inside it. Other organisms that acquire food using the process of phagocytosis are ciliates, that feed upon bacteria and algae using the phagocytosis process.
In multicellular organisms like human beings, professional phagocytes carry out the process of phagocytosis to eliminate foreign material or pathogens from the body. Macrophages, neutrophils, and dendritic cells are some of the professional phagocytes.
NOTE IT!
Are phagocytosis and pinocytosis the same?
A term similar to phagocytosis is pinocytosis, however, it is different from phagocytosis. The literal meaning of pinocytosis is ‘cell drinking’. This is because, unlike phagocytosis, herein the phagocytotic target is a liquid substance, which is engulfed during the process of pinocytosis. The liquid droplets that are ingested during the process of pinocytosis are not treated with lysozymes as being a liquid substance/droplet, it doesn’t need further breakdown.
Take the Phagocytosis – Biology Quiz!
References
- Lafuente, E. M., Niedergang, F., & Rosales, C. (2020). Editorial: Phagocytosis: Molecular Mechanisms and Physiological Implications. Frontiers in immunology, 11, 586918. https://doi.org/10.3389/fimmu.2020.586918
- Rosales, C., & Uribe-Querol, E. (2017). Phagocytosis: A Fundamental Process in Immunity. BioMed research international, 2017, 9042851. https://doi.org/10.1155/2017/9042851
- Stuart, L. M., & Ezekowitz, R. A. (2005). Phagocytosis: elegant complexity. Immunity, 22(5), 539–550. https://doi.org/10.1016/j.immuni.2005.05.002
- Uribe-Querol, E., & Rosales, C. (2020). Phagocytosis: Our Current Understanding of a Universal Biological Process. Frontiers in immunology, 11, 1066. https://doi.org/10.3389/fimmu.2020.01066
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.