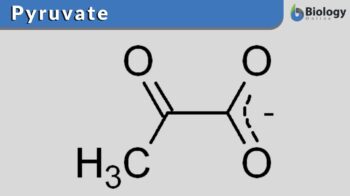
Pyruvate
n., [paɪˈɹuː.veɪt]
Definition: The carboxylate anion of pyruvic acid.
Table of Contents
Pyruvate Definition
Pyruvates have been often recognized as one of the most important molecules that are frequently represented at the intersection of many biochemical reactions and pathways. We can define pyruvate as a 2-oxo monocarboxylic acid anion whose conjugate base is pyruvic acid that arises from the deprotonation of carboxylic groups. It acts as the main metabolite and cofactor.
Pyruvate is commonly seen as one of the last products of glycolysis where a couple of its molecules can be produced by the relative breakdown of a single glucose molecule. The primary end product of glycolysis is pyruvate and water molecules, plus high-energy molecules, such as ATP and NADH.
Pyruvate is the end product of glycolysis, which is converted into acetyl coA that enters the Krebs cycle when there is sufficient oxygen available. But when the oxygen is insufficient, pyruvate is broken down anaerobically, such as in fermentation that creates lactate or ethanol as an end-product. Etymology: pyr(o)– + Latin ūva, grape (from its being produced by the dry distillation of racemic acid, originally derived from grapes) + -ate.
What is the major metabolic source of pyruvate?
The major metabolic source of pyruvate is glucose. Moreover, for the participation in the citric acid cycle, it is then transported to the mitochondrion (first, by converting pyruvate into acetyl CoA; more details below).
How does pyruvate enter the mitochondrion?
The pyruvate enters the mitochondria via the protein pyruvate translocase.
It has been reported that via fermentation, the pyruvate is transformed into lactate (e.g. in animals) or ethanol (e.g. in higher plants and yeasts). The said reaction occurs only in the absence of air or when the air demands outstrip the overall supply. Furthermore, glucose can be regenerated again by lactate and pyruvate. (Ref. 1) Similarly, the oxidation of pyruvate results in acetyl CoA formation.
Another term used is called pyruvate processing where the pyruvate is processed to release one molecule of carbon dioxide and the remaining two carbons are used to produce acetyl CoA.
Where does pyruvate oxidation occur?
The process of pyruvate oxidation occurs in the mitochondrial matrix. It must be remembered here that pyruvate oxidation is also known as pyruvate decarboxylation. Examples of biological processes that include such a process are the anabolic syntheses of amino acids and fatty acids. Likewise, scientists believe that it can influence the epigenetic modifications and nuclear activity, thus inducing an interface between the metabolic state and genome of the cell.
Pyruvate is produced by the bodies, once the sugar (glucose) is broken down and its molecular formula is C3H3O3– having a molecular weight of 87.5 g mol-1.
It is worth mentioning here that acetyl coenzyme A formation occurs when the molecule of pyruvate is oxidized via the Kreb cycle (citric acid cycle) reaction and oxaloacetate by anaplerotic reactions.
Pyruvate definition in biology
Pyruvate is the key intersection in metabolic pathways where it can be converted into fatty acids and energy through acetyl-CoA and carbohydrates by the process of gluconeogenesis and to ethanol and amino acid alanine. In this way, many metabolic processes are united that further enhance the significance of pyruvates in biology and biochemistry. (Ref. 2)
Pyruvate Structure
Pyruvate has a very simple chemical structure and it is the conjugate base of pyruvic acid. Three carbon molecules are composed of a ketone functional group and a carboxyl group present in the structure of pyruvate. The chemical formula of pyruvate is C3H3O3– and the chemical formula of pyruvic acid is C3H4O3 and C3H3O3 represents its deprotonated form. The carbon atom that forms the carboxylic acid is primarily written as the first carbon atom and along the carbon backbone, away from the carboxylic acid terminus, the number increases. Similarly, with the second carbon atom, which is also referred to as the a-carbon, the ketone group is attached and it is seen that this carbon is closest to the main functional group. Lastly, in the structure of pyruvate, the methyl group is attached to the third carbon. The structure of pyruvate can be seen in Figure 1.
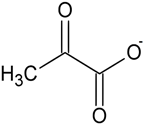
Pyruvate is the simplest alpha-keto acid, and it is also known as the a-keto propanoic acid via the official nomenclature of IUPAC. It has three atoms that act as hydrogen bond donors and hydrogen bond acceptors.
Pyruvate vs Pyruvic Acid
Are pyruvate and pyruvic acid the same? It is worth mentioning here that pyruvate can also be referred to as pyruvic acid thus both pyruvate and pyruvic acid are the same. The reason behind this is that when pyruvic acid is dissolved in water the proton present in the carboxylate group (-COOH) breaks leaving the charged pyruvate molecule and the proton. Thus, the proton, the pyruvate, and the pyruvic acid remain in equilibrium as elaborated in Figure 2. (Ref. 3) Although in any solution the concentrations of all three stated components can be seen the percentage of the concentrations will not change due to the fact the dissociation and the formation reactions are at equilibrium.
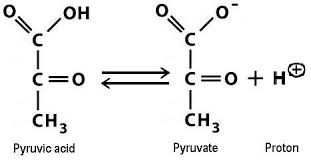
Question: What is pyruvic acid?
Answer: Pyruvic acid is a product of pyruvate C3H3O3 and a proton (H+). Additionally, it is believed that the pyruvic acid, like other keto acids, can also tautomerize from its ketone, form to its enol form, containing an alcohol molecule and a double bond. This is a very important procedure in the final step of glycolysis, a metabolic pathway that converts glucose into pyruvate.
Generation and Metabolism of Pyruvate
Where does pyruvate come from? The two most common methods through which the pyruvates are generated are by the metabolism of amino acids and another is the glycolytic pathway. It has been calculated that nearly ten percent of the energy needs of the human body are satisfied by the proteins, and only some amino acids are channeled into the cellular respiratory machinery through pyruvates. The amino acids that are directed are classified as glucogenic amino acids, while ketogenic amino acids are the one that usually results in acetyl-CoA formation or are otherwise referred to as acetoacetate. The pyruvate can also be regenerated by lactate that is reproduced by anaerobic fermentation as demonstrated above, through several enzymatic activities in the liver.
One of the other most important methods by which pyruvate is processed is referred to as glycolysis that initiates with six-carbon monosaccharide glucose. The primary steps of the biochemical process include the formation of fructose-6-phosphate in which glucose undergoes the process of phosphorylation and isomerization. Moreover, another phosphorylation reaction aids the breakdown of this glucose into three-carbon molecules stated as dihydroxyacetone phosphate (DHAP) and glyceraldehyde phosphate (G3P). These steps consume a couple of molecules of ATP for every molecule of glucose by acquiring the energy, thus transforming a molecule of hexose into two molecules of triose. Additionally, through enzyme-mediated catalysis, both DHAP and G3P isomers can be interconverted. Thus, it can be concluded that glyceraldehyde phosphate is converted into pyruvic acid where a total of five biochemical reactions are executed, liberating a single molecule of NADH and two molecules of ATP for each molecule of G3P.
It must be noted here that phosphoenolpyruvate abbreviated as PEP is the penultimate molecule that is produced via these chains of biochemical reactions, which is then phosphorylated ester of the pyruvate. Generally, a phosphate group is liberated by PEP so that pyruvate is formed and the released phosphate forms firstly ADP that finally forms ATP. The reaction is catalyzed by a very important enzyme known as pyruvate kinase (PK). Additionally, this reaction is irreversible and is the rate-determining step in the process of conversion of glucose into pyruvate as this is one of the slowest steps in the chain reaction.
Another handy process to produce pyruvate is the metabolism of amino acids where the six distinct amino acids, namely serine, glycine, alanine, threonine, cysteine, and tryptophan, can be metabolized to form pyruvate. Among all the six amino acids, the easiest to transform are serine and alanine as they are three carbon atoms. In these reactions, a single group of enzymes, transaminases, catalyzes the replacement of the functional group of amines with a ketone.
Although cysteine is also a three-carbon atom its transformation into pyruvate includes an additional step where the sulfur atom is removed. Furthermore, there are only two carbon atoms in glycine, thus, before undergoing the process of deamination, it is transformed into three-carbon amino acid (typically, serine), which hastens its conversion into pyruvate.
The conversion of tryptophan also follows the same procedure — wherein three alkyl groups of tryptophan are initially converted into alanine and then by the action of alanine transaminase enzyme gets transformed into a molecule of pyruvate.
Lastly, the amino acid, threonine, follows the longest path to convert into pyruvate. In the process, initially, it gets converted into glycine, and then to serine before being acted on by serine dehydratase.
Functions of Pyruvate
The primary function of pyruvate is to serve as the transporter of carbon atoms into the mitochondrion for complete oxidation into carbon dioxide. In the cytoplasm, at the end of the process of glycolysis, the molecules of pyruvate that are generated from the sugar are sent to the matrix of the mitochondria via a couple of proteins that are mitochondrial pyruvate-carriers 1 and 2 (i.e. MCP1 and MCP2, respectively).
Pyruvate dehydrogenase, which is a significant complex of multi-enzyme, catalyzes the reactions of oxidation and decarboxylation for the production of acetyl coenzyme A referred to as acetyl-CoA. The first enzyme in the said complex is known as pyruvate dehydrogenase where the carboxylic group is removed from the molecule thus leaving a two-carbon molecule behind that consists of a carbonyl group and a methyl group. Furthermore, the second and the third enzymes of PDC oxidizes the already produced carbonyl group and through a thioester linkage, accelerate the covalent linkage to CoA. It is worth noting here that the thioester that is produced can be added to water along with the release of energy.
Scientists have recently diverted the attention of pyruvates in affecting the genome-wide acetylation of the molecules of histone. The epigenetic alterations that can transform the whole transcriptional activity of the cells, mitosis, and the cell cycle are known as histone acetylation. The main condition to achieve such acetylation is the availability and presence of acetyl-CoA. The two possible ways to produce acetyl-CoA is via PDC in the nucleus or by the transfer of enzyme complex to the nucleus from the mitochondria. The external environment, cell cycle, the availability of nutrients and the growth factors are some of the factors that affect the concentration of acetyl-CoA in the nucleus. It has also been observed that apart from the acetyl-CoA, pyruvate kinase is another enzyme that is present in the nucleus and is associated with pyruvate metabolism. The prime advantage of this kinase is that it generates the pyruvate from PEP in the last formed reactions of glycolysis. Similarly, the relevant literature states that this kinase plays a vital role in the nucleus and phosphorylating nuclear proteins. Additionally, if aerobic respiration is not possible the fermentation of pyruvate to lactic acid can be seen.
What must pyruvic acid be converted to before it can enter the citric acid cycle?
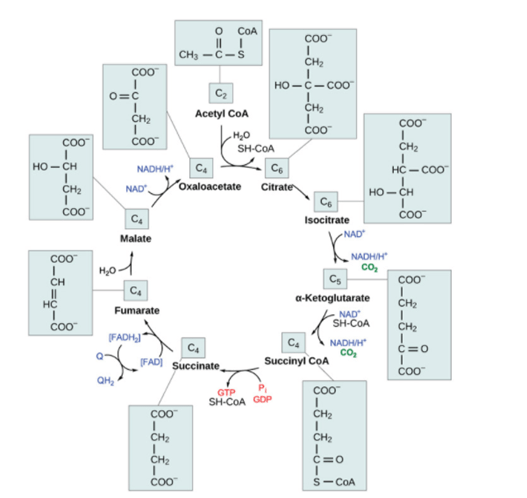
It is the conversion of pyruvate into acetyl CoA. It is through this that it can enter the citric acid cycle. Generally, there are seven important steps of the citric acid cycle (which is also known as the Krebs cycle as mentioned previously).
- In the first step, the condensation reaction occurs, where the two-carbon acetyl group combines with the four-carbon oxaloacetate molecule resulting in the formation of a six-carbon molecule of citrate.
- In the next stage, the molecule of citrate rapidly loses a single molecule of water as here, citrate gets converted into isocitrate which is its isomer.
- In step three, the oxidation of isocitrate occurs, resulting in the formation of the five-carbon molecule.
- In stages three and four, the reactions of oxidation and decarboxylation occur.
- In the next stage, a phosphate group is produced for the coenzyme A, thus leading to the formation of a very high energy bond.
- The next step in the overall process of dehydration where succinate is converted into fumarate.
- The last step of the citric acid cycle is the addition of water in the fumarate and malate is produced. Moreover, here in the last step, the citric acid cycle regenerates, oxaloacetate by the oxidation of malate. Here, it has been seen that yet another molecule of NADH is successfully formed.
The overall cycle of citric acid has been shown in Figure 3. (Ref. 4)
Biological Importance of Pyruvate
There are many biological and biochemical functions of pyruvates.
Question: What is the role of pyruvate in cellular respiration?
Answer: Pyruvates generally supply energy to the cells through the citric acid cycle facilitating cellular respiration.
Question: What is the role of pyruvic acid in fermentation?
Answer: The main role of pyruvic acid in fermentation is that it provides the pyruvate and NADH from glycolysis.
Apart from our previous discussion of the essential role of pyruvate in cellular respiration, it has been used in the medical and aesthetics industry. For instance, pyruvates are sold as weight loss supplements. It also seems to help to smoothen the human skin by decreasing the extent of wrinkles, reducing the dark spots that are found in the skin due to aging, and lengthy exposure of skin to the sun. the most recent research suggests that the small intake of sodium pyruvate improves the working capacity of the livers in patients who regularly consume extensive proportions of alcohol. Along with improving the functionality of livers, the performance of the lungs can also be enhanced in patients that are suffering from Chronic Obstructive Pulmonary Disease (COPD). The early research also suggests that congestive heart failure (CHF) can be avoided by taking a very minute amount of pyruvate as the solution. Lastly, the skin shedding in the majority of the population with flaky and scaly skin can be increased. Thus, it can be concluded that there are many examples of practical biological pyruvate functions that can easily be observed in our daily life.
Try to answer the quiz below to check what you have learned so far about pyruvates.
References
(1) Ruthenium-Catalyzed Diastereoselective Synthesis of Fully Substituted Pyrrolidines from Anilines and Diazo Pyruvates. (2020). Organic Letters. https://pubs.acs.org/doi/10.1021/acs.orglett.0c00846
(2) 5.3B: Pyruvic Acid and Metabolism. (2017, May 8). Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Microbiology/Book%3A_Microbiology_(Boundless)/5%3A_Microbial_Metabolism/5.03%3A_Catabolism/5.3B%3A_Pyruvic_Acid_and_Metabolism
(3) Moran, L. (2007, April 17). Pyruvate. Sandwalk. https://sandwalk.blogspot.com/2007/04/pyruvate.html
(4) Pyruvate Oxidation | Biology for Majors I. (2020). Lumenlearning.com. https://courses.lumenlearning.com/wm-biology1/chapter/reading-pyruvate-oxidation/
©BiologyOnline.com. Content provided and moderated by BiologyOnline Editors.