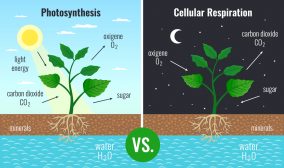
Acid
(Science: chemical, chemistry) a fundamental category of many compounds whose water-based solutions have a sour taste, turn blue litmus paper red and can combine with metals to form salts.
They are chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization).
An extension of the term includes substances dissolved in media other than water. Specific types of acids include:
Arrhenius acid: any chemical that increases the number of free hydrogen ions (H-) when added to a water-based solution. The more free hydrogens produced, the stronger the acid.
Bronsted or Bronsted-Lowry acid: any chemical that acts as a proton donor in a chemical reaction.
lewis acid: any chemical that accepts two electrons to form a covalent bond during a chemical reaction.
Containing acid; an acid taste.Any of various water-soluble compounds having a sour taste and capable of turning litmus red and reacting with a base to form a salt.Substances that have a ph of lower than 7 (neutral) that can dissolve in water.
Dictionary > Acid