Amphipathic
adj., [ˌam.fɪˈpaθ.ɪk]
Definition: Having both hydrophobic and hydrophilic groups
Table of Contents
Amphipathic Definition
Amphipathic is a word used to describe a chemical compound containing both polar (water-soluble) and nonpolar (not water-soluble) portions in its structure. It may also relate to a chemical compound having both hydrophobic and hydrophilic regions. In biology, amphipathic molecules are important in the formation of biological membranes and micelles. Through them, the plasma membrane, in particular, is able to create an effective selective barrier so that not all substances can enter or exit the cell. Instead, some of them need transport mechanisms. This is essential in order to regulate their concentration inside the cell, and this, in turn, is crucial in maintaining homeostasis.
Etymology
The term amphipathic came from Greek amphis, meaning “both” and pathy, from Greek pátheia, meaning “suffering”, “feeling”. Synonyms: amphiphilic.
Structure
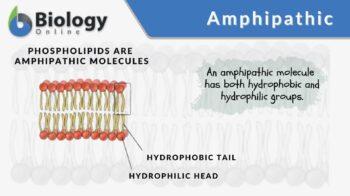
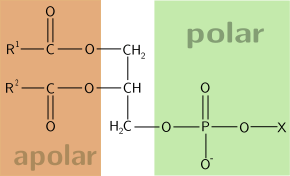
The term amphipathic is a descriptive word for a substance or a chemical compound that possesses both hydrophobic and hydrophilic portions in its structure. The hydrophobic portion is typically a large hydrocarbon moiety CH3 (CH2)n, with n > 4). This portion is nonpolar and lipophilic. The hydrophilic portion is either charged or uncharged polar functional group. The charged group may be anionic or cationic. The anionic group carries a negative charge and could be in the form of carboxylates (RCO2–), sulfates (RSO4–), sulfonates (RSO3–), and phosphates. The cationic group, in turn, carries a positive charge. An example is ammonium (RNH3+). An amphipathic compound may have several hydrophobic components — or several hydrophilic components — or both.
Characteristics
Because an amphipathic compound is comprised of two different constituents, its parts may react in opposing ways. For instance, its hydrophilic portion will readily react with polar molecules. Thus, it can be dissolved using polar solvents, such as water. In contrast, the hydrophobic portion will not react with polar molecules. Rather, it repels them. And so, unlike the hydrophilic portion, the hydrophobic part will not dissociate into ions in the presence of water. Other polar molecules would not be able to react with this portion but certain non-polar organic solvents would. Thus, a solution containing both aqueous and non-polar organic solvents will be able to separate an amphipathic compound into two partitions.
Amphipathic biomolecules
An amphipathic chemical compound is called an amphiphile. Many biomolecules are amphipathic, such as proteins, phospholipids, cholesterol, glycolipids, bile acids, and saponins.
Amphipathic proteins
Amphipathic proteins are comprised of polar and nonpolar sequences of amino acids. For instance, a protein may be made up of hydrophilic portions of polar (charged) amino acids (e.g. Asp-Ser, Tyr-Glu) and hydrophobic portions of nonpolar amino acids (e.g Gly-Pro, Ile-Pro-Met). An example is the membrane proteins found in biological membranes. (1)
Their amphipathic nature enables them to insert themselves into the hydrophobic, nonpolar region of a biological membrane and at the same time expose their hydrophilic portion to the polar aqueous medium. And these protruding hydrophilic portions of the protein can interact with polar molecules. Most of these amphipathic proteins are capable of these seemingly opposing interactions because of their amphipathic helices.
An amphipathic helix is a protein helix conformation characterized by the presence of opposing faces. The face oriented along the long axis of the helix is hydrophilic whereas the opposite face is hydrophobic. Thus, it can segregate the hydrophobic and hydrophilic domains of a protein. Furthermore, it allows self-association and protein-protein interactions. Amphipathic helices are a common structural feature of proteins. Examples of proteins with this conformation are ion channel membrane proteins, lung surfactant proteins, and apolipoprotein. (2)
Phospholipids

A phospholipid is another amphipathic molecule. It is a type of lipid comprised of glycerol bound to two fatty acids and a phosphate group. The glycerol with an attached negatively charged phosphate group is the hydrophilic head of a phospholipid. The phosphate group may be further bound to hydrogen, choline, serine, ethanolamine, or inositol, thus, diversifying into phosphatidic acid, phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, and phosphatidylinositol phospholipids, respectively. The two long fatty acid chains are the lipophilic hydrophobic tail of the phospholipid.
The amphipathic nature of phospholipids made the latter an essential component of biological membranes. The plasma membrane, for instance, is largely made up of two layers of phospholipids. As amphipathic, phospholipids can interact with various molecules depending on polarity. The phospholipid heads interact readily with water and other polar molecules. The phospholipid tails, in contrast, tend to avoid water and other polar interactions. Thus, phospholipids in water will aggregate by orienting their tails toward each other while exposing their heads to the aqueous environment. In fact, it is the amphipathic nature of phospholipids that helps form the bilayer structure of the plasma membrane. The phospholipid tails orient themselves such that their tails line up internally of the plasma membrane while the phospholipid heads face the exterior.
Cholesterol
Another amphiphile is cholesterol. It is made up of the hydrophilic hydroxyl group (-OH) and the hydrophobic bulky steroid and hydrocarbon chain. Cholesterol is found in the animal plasma membranes. Its hydrophilic portion interacts with the aqueous medium and with the polar heads of the phospholipid. Its hydrophobic portion, in turn, is embedded in the membrane alongside the hydrophobic tails of the phospholipids and the nonpolar fatty acid chains of other lipids.
Glycolipids
Glycolipids are amphipathic compounds since they are made up of hydrophilic sugar group(s) covalently linked to a hydrophobic lipid tail. They are also present in the plasma membrane. The carbohydrate component extends to the cell exterior while the lipid component is embedded in the lipid bilayer. The sugar residues exposed to the exterior of the cell allow carbohydrate-carbohydrate interactions.
Bile acids
Bile acids have a steroid structure comprised of four rings and a side chain ending in carboxylic acid and hydroxyl groups. Salts of bile acids can aggregate around the droplets of lipids and form micelles. When aggregated, they act as a surfactant. They emulsify lipids. This prevents fat droplets from aggregating into larger fat particles.
Saponins
Saponins are amphipathic glycosides that are abundant in plants. The fundamental structure is a hydrophilic glycoside moiety and a hydrophobic triterpene or steroid derivative.3 Plants produce them, presumably, to discourage too much herbivory. They are bitter, and therefore, make plants less palatable.
Biological Functions
The amphipathic nature of biomolecules is essential to their biological roles. Biological membranes and micelles form as amphipathic molecules organize themselves. Because they have opposing components, they are able to react distinctively with diverse molecules.
Membrane formation
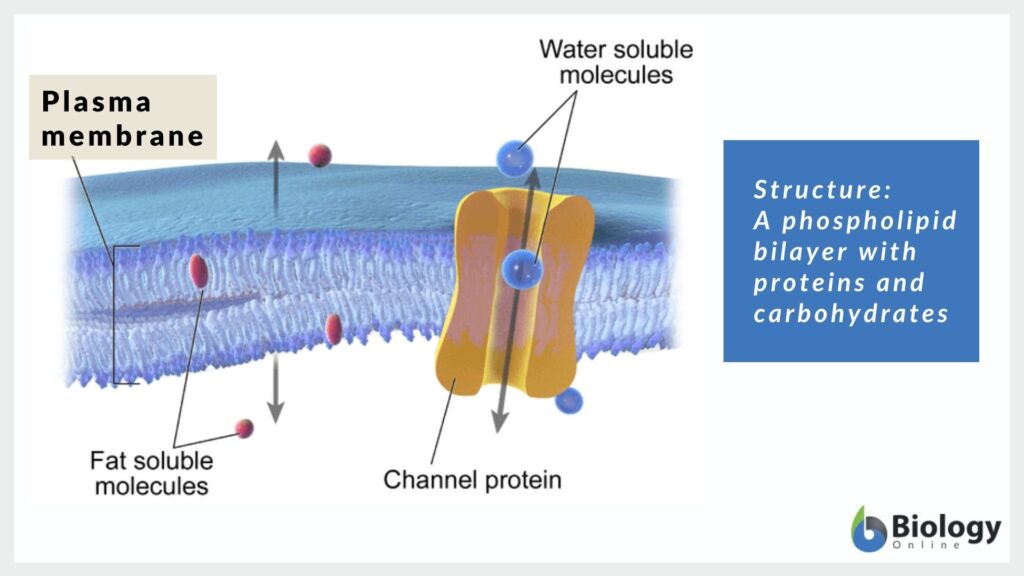
The plasma membrane is a classic example of a biological structure made up of biomolecules whose amphipathic features enable the plasma membrane to become selectively permeable. In particular, phospholipids occupy a huge proportion of the plasma membrane. These lipid molecules have hydrophilic and hydrophobic components that when they orient themselves create a lipid bilayer. The phospholipid tails form the interior of the lipid bilayer. Then, their phospholipid heads are placed on the exterior. This spatial arrangement is crucial to the movements of molecules across the plasma membrane.
Small nonpolar molecules can readily diffuse to their concentration gradient across the membrane whereas polar molecules will be prohibited from doing so. The hydrophobic lipid bilayer forms a barrier between the interior and the exterior of the cell. Thus, the transport of polar molecules needs to be modulated.
Polar molecules, such as water and certain proteins, and ions need a transporter in the plasma membrane to cross. This is the function of membrane proteins. Since membrane proteins are also amphipathic molecules, they can interact with the hydrophobic lipid bilayer and thereby insert themselves in the membrane. At the same time, they provide a transport mechanism by which polar and charged molecules pass through. Thus, while the lipid bilayer prevents them to enter or leave the cell, the membrane proteins are their vehicles for entry and exit. This is important for the cell to ensure that the cytosolic components are kept at optimal levels, thereby maintaining homeostasis.
The selective permeability of the plasma membrane is a fundamental feature of biological membranes. Thus, the membraned organelles such as the nucleus, endoplasmic reticulum, Golgi apparatus, mitochondria, chloroplasts, and vesicles are able to similarly regulate the passage of molecules.
Cholesterol molecules are another essential amphiphile. They are present in the plasma membrane of animal cells and are responsible for membrane fluidity and structural integrity of animal cells. Because of them, animal cells do not need a cell wall. Their presence in the animal cell membrane ensures cellular integrity. While they keep the membrane stable, they also allow an animal cell to alter its shape and move. They are also involved in intracellular transport, selective permeability, cell signaling, and nerve conduction.
Glycolipids are another plasma membrane component. They provide stability to the cell. They also permit cell-to-cell interactions. They enable tissue formation by cell adhesion. Also, they facilitate cellular recognition, which is essential in immunologic functions.
Micelle formation
A micelle is an aggregate of surfactant molecules wherein the hydrophilic head regions face the aqueous solution and the hydrophobic tail regions are oriented towards the center. Thus, it is often spherical in shape. Due to the amphipathic nature of bile acids, they are able to form micelles. Bile acid-containing micelles aid in lipid digestion. They bring the lipids near the intestinal brush border membrane to prompt fat absorption.4
Try to answer the quiz below to check what you have learned so far about amphipathic.
Further Reading
References
- Membrane Proteins. (2019). Retrieved from Miami.edu website: http://fig.cox.miami.edu/~cmallery/150/memb/amphipathic.htm
- Epand, R. (1993). The Amphipathic helix. Boca Raton: CRC Press.
- “Saponins”. Cornell University. (14 August 2008). Retrieved from http://www.ansci.cornell.edu/plants/toxicagents/saponin.html
- Hofmann, A. F. & Borgström, B. (February 1964). “The intraluminal phase of fat digestion in man: the lipid content of the micellar and oil phases of intestinal content obtained during fat digestion and absorption”. J. Clin. Invest. 43 (2): 247–57.
- Molecular Expressions Cell Biology: Plasma Membrane. (2019). Retrieved from Fsu.edu website: https://micro.magnet.fsu.edu/cells/plasmamembrane/plasmamembrane.html
© Biology Online. Content provided and moderated by Biology Online Editors