Carbohydrate
noun
plural: carbohydrates
[car·bo·hy·drate, kɑːbəʊˈhaɪdɹeɪt]
Definition: any of the group of organic compounds consisting of carbon, hydrogen, and oxygen, usually in the ratio of 1:2:1, hence the general formula: Cn (H2O) n
Table of Contents
Carbohydrate Definition
A biomolecule refers to any molecule that is produced by living organisms. As such, most of them are organic molecules. The four major groups of biomolecules include amino acids and proteins, carbohydrates (especially, polysaccharides), lipids, and nucleic acids. A carbohydrate refers to any of the group of organic compounds consisting of carbon, hydrogen, and oxygen, usually in the ratio of 1:2:1, hence the general formula: Cn (H2O) n. Carbohydrates are the most abundant among the major classes of biomolecules.
Characteristics of Carbohydrates
Carbohydrates are organic compounds. An organic compound is a compound that, in general, contains carbon covalently bound to other atoms, especially Carbon-Carbon (C-C) and Carbon-Hydrogen (C-H). Carbohydrates are an example of the many types of organic compounds. Its four major element constituents are carbon, hydrogen, oxygen, and nitrogen. Most of them follow the general formula: Cn (H2O) n, from where they derive their name, carbohydrates (which means hydrates of carbon). That is because the ratio of hydrogen atoms to oxygen atoms is often 2:1. However, not all carbohydrates follow this formula. In essence, they are organic compounds that are aldehydes or ketones with many hydroxyl groups added, usually on each carbon atom not part of the aldehyde or ketone functional group.
Carbohydrates are energy-rich biomolecules. They are one of the major nutrients required by many living organisms because they provide the body with a source of chemical energy. ATP is chemical energy produced via a series of metabolic processes in cellular respiration. In brevity, glucose (a monosaccharide) is “churned” to extract energy, primarily, in the form of ATP. Firstly, a series of reactions leads to the conversion of glucose to pyruvate. Then, it uses pyruvate, converting it into acetyl coenzyme A for oxidation via an enzyme-driven cyclic reaction called Krebs cycle. Finally, a cascade of reactions (redox reactions) involving the electron transport chain leads to the production of ATPs (via chemiosmosis).1 Glucose molecules used in glycolysis are derived from a carbohydrate-containing diet. Complex carbohydrates are broken down into simpler monosaccharides, such as glucose, by saccharification during digestion.
Carbohydrates are one of the major sources of nutrition in animals, including humans. However, many other carbohydrates are in the form of fibers. And as fiber, it is not readily digested by humans. Typically, fibrous carbohydrates include mucilages, pectins, gums, and insoluble components, such as those found in lignin and cellulose. Ruminants, such as cattle, sheep, deer, and goats, are capable of digesting plant materials that are otherwise indigestible to humans. Certain symbiotic bacteria (e.g. Ruminococcus, Fibrobacter, Streptococcus, Escherichia ) reside in their rumen that can degrade cellulosic materials into simpler carbohydrates for ruminants.
Classification of Carbohydrates
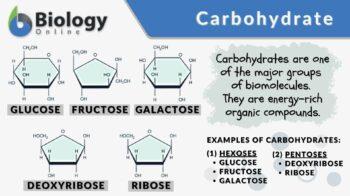
Many carbohydrates are polymers. A polymer is a compound made up of several repeating units (monomers) or protomers and produced by polymerization. Saccharide is the structural (monomeric) unit of carbohydrates. Carbohydrates may be classified into monosaccharides, disaccharides, oligosaccharides, and polysaccharides based on the number of saccharide units.
The most fundamental type is the simple sugars called monosaccharides. These simple sugars can combine with each other to form more complex types. Examples are glucose, galactose, and fructose. The combination of two simple sugars is called a disaccharide. Examples are sucrose, maltose, and lactose. Carbohydrates consisting of three to ten simple sugars are called oligosaccharides. Examples are raffinose, maltotriose, and maltotetraose. Carbohydrates made up of several saccharide units are called polysaccharides. When a polysaccharide is made up of saccharide units of the same type it is referred to as a homopolysaccharide (or homoglycan) whereas a polysaccharide is comprised of more than one type of saccharide it is called heteropolysaccharide (or heteroglycan). Examples of polysaccharides are starch, cellulose, and glycogen.
In terms of nutrition, carbohydrates are classified into two major food groups: simple and complex. Simple carbohydrates — sometimes referred to as simply “sugar” — are those that are readily digested and serve as a rapid source of energy. Complex carbohydrates are those that need more time to be digested and metabolized. They often are high in fiber, and unlike simple carbohydrates, they are less likely to cause spikes in blood sugar.
Functions of Carbohydrates
As noted earlier, one of the major functions of carbohydrates is to provide energy to organisms. Monosaccharides, in particular, are the main source of energy for metabolism. When they are not yet needed, they are converted into energy-storing polysaccharides, such as starch in plants and glycogen in animals.
In plants, starch is abundant in amyloplasts inside the cells of various plant organs, e.g. fruits, seeds, rhizomes, and tubers. In animals, glycogen is stored in the liver and in the muscle cells.
Apart from that, carbohydrates are also important structural components.
At the cellular level, polysaccharides (e.g. cellulose) are constituents of the cell walls of plant cells and many algae. Cells without cell walls are more prone to structural and mechanical damage. In plants, the cell wall prevents the cell from bursting into a hypotonic solution.
The osmotic pressure drives water to diffuse into the cell. The cell wall resists the osmotic pressure and thereby prevents the cell from bursting.
In bacterial cell walls, the structural carbohydrate is murine whereas in fungi the polysaccharide chitin is a cell wall component. Some bacteria have a polysaccharide “capsule” that helps them to evade detection by immune cells. Some animals have chitin exoskeletons that provide strength and protection in soft-bodied animals.
Nucleic acids, such as RNA and DNA, have a sugar component in them, i.e. ribose and deoxyribose, respectively. Many other biological molecules have sugar components as well, such as glycoproteins, glycolipids, proteoglycans, which in turn carry out vital roles, e.g. in immune response, detoxification, blood clotting, fertilization, biological recognition, etc.
Common Biological Reactions Involving Carbohydrates
Below are some of the common biological reactions involving carbohydrates.
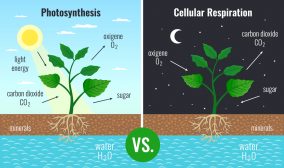
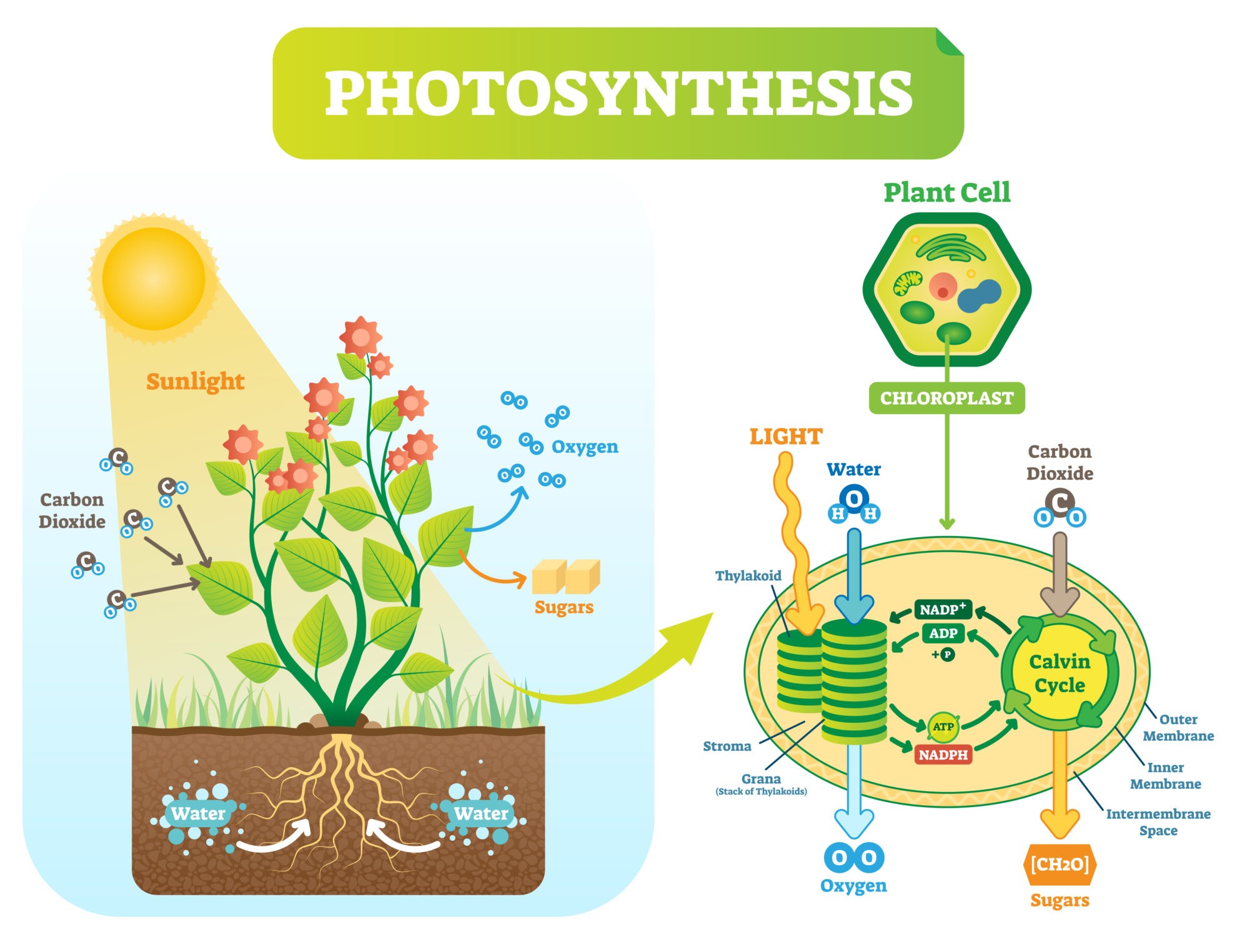
Photosynthesis
In plants and other photosynthetic autotrophs, the synthesis of simple sugars (e.g. glucose) is done through photosynthesis. The process uses carbon dioxide, water, inorganic salts, and light energy (from sunlight) captured by light-absorbing pigments, such as chlorophyll and other accessory pigments to produce glucose, water, and oxygen molecules.
Dehydration synthesis
Monosaccharide forms carbohydrates by joining together in glycosidic bonds through a process called dehydration synthesis. In the formation of a disaccharide, for instance, the joining of two monosaccharides results in the release of water as a by-product. Similarly, polysaccharides are formed from a long chain of monosaccharide units by further dehydration process. Starch and glycogen that are formed serve as energy-rich molecules. These complex carbohydrates are degraded into simpler forms (e.g. glucose) when the body requires more energy. This process is called saccharification.
Saccharification
The process wherein complex carbohydrates are degraded into simpler forms, such as glucose, is called saccharification. It entails hydrolysis. In humans and other higher animals, this involves enzymatic action. In the mouth, glucose-containing complex carbohydrates are broken down into simpler forms through the action of salivary amylase. In the small intestine, the digestion of complex carbohydrates is continued. Enzymes such as maltase, lactase, and sucrase break down disaccharides into monosaccharide constituents. Glucosidases are another group of enzymes that catalyze the removal of the terminal glucose from a polysaccharide comprised chiefly of long chains of glucose.
Assimilation
Monosaccharides from the digested carbohydrates are absorbed by the epithelial cells of the small intestine. The cells take them up from the intestinal lumen through the sodium ion-glucose symport system (via glucose transporters or GluT). GluTs are proteins facilitating the transport of monosaccharides, such as glucose, into the cell. Next, they are released into the capillaries by facilitated diffusion. Cells of tissues take them up from the bloodstream again via GluTs. When inside the cell, glucose is phosphorylated to trap it inside the cell. As an effect, glucose-6-phosphate may be used in any of the following metabolic pathways: (1) glycolysis, to synthesize chemical energy, (2) glycogenesis, where glucose is brought to the liver via the vena portae to be stored as cellular glycogen, or (3) Pentose phosphate pathway to form NADPH for lipid synthesis and pentoses for nucleic acid synthesis.
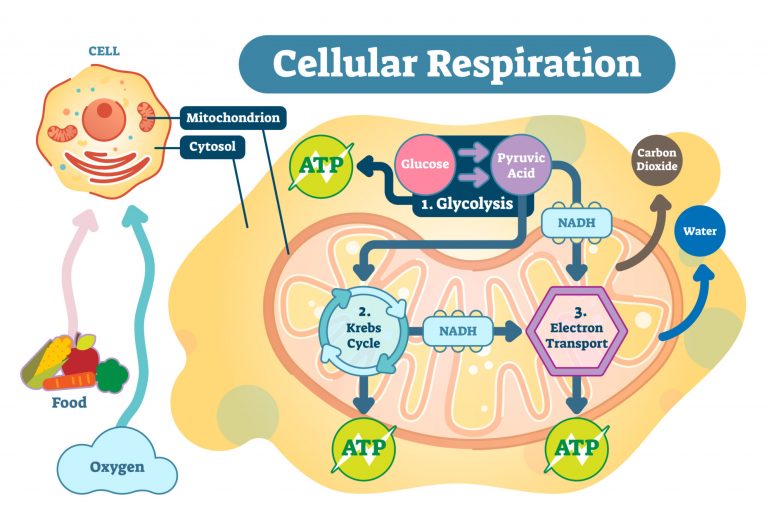
Cellular respiration
Glucose is metabolized by the cell in a process called cellular respiration. The major steps or processes of cellular respiration are (1) Glycolysis, (2) Krebs cycle, and (3) Oxidative phosphorylation. In the initial step (i.e. glycolysis), a series of reactions in the cytosol results in the conversion of a monosaccharide, often glucose, into pyruvate, and the concomitant production of a relatively small amount of high-energy biomolecules, such as ATP. NADH, an electron-carrying molecule, is also produced. In the presence of sufficient oxygen, the pyruvate from glycolysis is converted into an organic compound to be completely oxidized inside the mitochondrion. Electron carriers (e.g. NADH and FADH2) shuttles the electrons down the electron transport chain. A series of redox reactions occurs along the chain and culminates in the final electron acceptor, i.e. the molecular oxygen. More ATP is produced via a coupling mechanism through chemiosmosis in the inner mitochondrial membrane.
From glycolysis alone, the net ATP is two (from substrate-level phosphorylation). By oxidative phosphorylation, the net ATP is about 34. Thus, the total net ATP per glucose is about 36.2 In the absence or insufficiency of oxygen, anaerobic catabolism occurs (e.g. by fermentation). Fermentation is an anaerobic process that produces ATP from glycolysis. However, instead of shuttling the electrons in the electron transport chain, NADH transfers the electrons to pyruvate, restoring the NAD+ that sustains glycolysis.2 The total ATPs produced per glucose from fermentation is only about two.
Gluconeogenesis
Gluconeogenesis seems like the reverse of glycolysis in a way that glucose is converted into pyruvate whereas, in gluconeogenesis, pyruvate is converted into glucose. In essence, gluconeogenesis is a metabolic process wherein glucose is generated from non-carbohydrate precursors, e.g. pyruvate, lactate, glycerol, and glucogenic amino acids. In humans and many other vertebrates, gluconeogenesis takes place mainly in the liver cells. It often occurs during periods of fasting, low-carbohydrate diets, or intense exercise. Cytologically, the process begins in the mitochondria and then ends in the lumen of the endoplasmic reticulum. Glucose formed from hydrolyzing glucose-6-phosphate by the enzyme glucose-6-phosphatase is shuttled from the endoplasmic reticulum into the cytoplasm.
Glycogenesis
Glycogenesis is the metabolic process of producing glycogen from glucose for storage mainly in liver and muscle cells in response to the high glucose levels in the bloodstream. Short polymers of glucose, especially exogenous glucose, are converted into long polymers to be stored inside the cells, mainly in the liver and the muscle. When the body requires metabolic energy, glycogen is broken down into glucose subunits through the process of glycogenolysis. Thus, glycogenesis is the opposite process of glycogenolysis.
Glycogenolysis
Glycogenolysis is the process of breaking down stored glycogen in the liver so that glucose may be produced for use in energy metabolism. Stored glycogen in the liver cells is broken down into glucose precursors. A single glucose molecule is cut off from the glycogen and is converted into glucose-1-phosphate, which in turn, is transformed into glucose-6-phosphate that can enter glycolysis.
Pentose phosphate pathway
Pentose phosphate pathway is a glucose metabolic pathway in which five-carbon sugars (pentoses) and NADPH are synthesized in the cytosol. The pentose phosphate pathway serves as an alternative metabolic route in the breakdown of glucose. In animals, it occurs in the liver, adrenal cortex, adipose tissues, testis, etc. This pathway is the main metabolic pathway in neutrophils. Thus, congenital deficiency in the pathway produces sensitivity to infection. In plants, part of the pathway functions in the formation of hexoses from carbon dioxide in photosynthesis.
Leloir pathway (Galactose metabolism)
In this metabolic pathway, galactose enters glycolysis by first being phosphorylated via the enzyme galactokinase and then converted into glucose-6-phosphate. Galactose is derived from lactose (milk sugar comprised of a glucose molecule and a galactose molecule).
Fructose 1-phosphate pathway
In this metabolic pathway, fructose, instead of glucose, enters glycolysis. Nevertheless, fructose needs additional steps prior to entering glycolysis. In animals, it occurs in the muscles, the adipose tissues, and the kidney.
Glucoregulation
Proper metabolism of carbohydrates is essential for the proper assimilation and catabolism of carbohydrates within the organism. The maintenance of the steady levels of glucose in the body is called glucoregulation. Hormones, such as insulin and glucagon from the pancreas, regulate the proper metabolism of glucose. Blood sugar refers to the amount of glucose circulating in the body. When there is a low blood glucose level, glucagon is released. Conversely, a high blood glucose level stimulates the release of insulin. Insulin regulates the metabolism of carbohydrates (as well as fats) by promoting the uptake of glucose from the bloodstream into the skeletal muscles and fat tissues to be stored as glycogen for later use in glycogenolysis. Glucagon, in turn, acts by stimulating the production of sugar. In particular, it causes the stored glycogen in the liver to be converted into glucose that will be released into the bloodstream.
Improper carbohydrate metabolism may result in certain metabolic diseases or disorders, e.g. diabetes mellitus, lactose intolerance, galactosemia, glycogen storage disease, and fructose malabsorption.
Try to answer the quiz below to check what you have learned so far about Carbohydrates.
Further Reading
- biomolecule
- macromolecules
- nutrition
- photosynthesis
- monosaccharide
- disaccharide
- oligosaccharide
- polysaccharide
- starch
- glycogen
- glycolysis
- gluconeogenesis
- glycogenolysis
- glycogenesis
- pentose phosphate pathway
Reference
- Gonzaga, M. V. Mitochondrial DNA – hallmark of psychological stress – Biology Blog & Dictionary Online. (2018, September 29). Retrieved from ://www.biologyonline.com/mitochondrial-dna-hallmark-of-psychological-stress/ Link
- Campbell, N. A. (1996). Biology. California: The Benjamin/Cumming Publishing Company, Inc. pp. 159-ff.
Notes
More info relating to carbohydrates and their role in our diet can be found in the developmental biology tutorial investigating a balanced diet.
© Biology Online. Content provided and moderated by Biology Online Editors