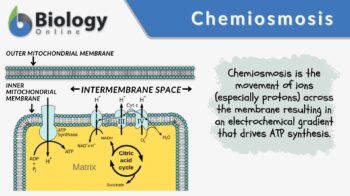
Chemiosmosis
n., [ˌkɛmɪɒzˈməʊsɪs]
Energy-coupling mechanism: to move ions (e.g. protons) to the other side of the membrane resulting in the generation of an electrochemical gradient and in the subsequent return of ions by downhill movement with the help of the membrane proteins
Table of Contents
Chemiosmosis Definition
What is chemiosmosis? In biology, chemiosmosis refers to the process of moving ions (e.g. protons) to the other side of a biological membrane, and as a result, an electrochemical gradient is generated. This can then be used to drive ATP synthesis. The gradient also incites the ions to return passively with the help of the proteins embedded in the membrane. By “passively”, it means that the ions will move from an area of higher concentration to an area of lower concentration.
This process is similar to osmosis where water molecules move passively. In the case of chemiosmosis, though, it involves the ions moving across the membrane; in osmosis, it is the water molecules. Nevertheless, both processes require a gradient. In osmosis, this is referred to as an osmotic gradient. The differences in the pressures between the two sides of the membrane drive osmosis. As for chemiosmosis, the movement of ions is driven by an electrochemical gradient, such as a proton gradient.
Not only is chemiosmosis similar to osmosis. It is also similar to other forms of passive transport, such as facilitated diffusion. It employs a similar principle. The ions move downhill. Also, the molecules are transferred to the other side of the membrane with the help of membrane proteins. Membrane proteins help the ions to move across since the membrane is not readily permeable to ions, basically because of its bilipid feature. READ: Plasma Membrane Structure
These proteins in the membrane facilitate their movement by acting as a temporary shuttle or by serving as a channel or a passageway. Chemiosmosis uses membrane proteins to transport specific ions. Furthermore, it does not require chemical energy (e.g. ATP) as opposed to an active transport system that does. In chemiosmosis, the formation of an ion gradient leads to the generation of potential energy that is sufficient to drive the process.
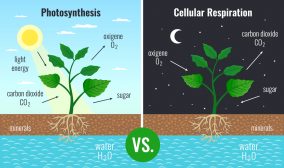
Where does chemiosmosis occur? In eukaryotes, it occurs in the mitochondria during cellular respiration and in the chloroplasts during photosynthesis. Prokaryotes lack these organelles and therefore chemiosmosis will occur in their cell membrane.
Chemiosmosis is a biological process wherein ions (usually protons, H+) are moved to the other side of the membrane resulting in the generation of an electrochemical gradient that can be used to drive ATP synthesis. Etymology: “chemi-“, relating to chemicals, and “osmosis”, from Ancient Greek “ὠσμός” (“ōsmós”), meaning “push”. Variant: chemosmosis. See also osmosis, chemiosmotic theory.
.
Chemiosmotic Theory
According to the chemiosmotic theory, chemiosmosis is driven by an electrochemical proton gradient essential during the production of ATP. This theory was proposed by Peter D. Mitchell (1920 – 1992), a British biochemist. (Ref. 1)
Mitchell’s hypothesis, however, was not accepted instantly until a substantive groundwork on proton pumping was laid. The discovery of ATP synthase and the pH difference across the thylakoid had the bioenergetics community consider the validity of his hypothesis. (Ref. 2)
In the 1960s, he knew about the phenomenon of membrane potential in which the inner side of the membrane being negative relative to its environment. (Ref. 1) ATP was also already recognized at that time as the cell’s major energy currency. However, how living organisms produce ATP biologically was not well established.
The mitochondria have long been known as the organelles responsible for ATP synthesis. How these organelles generate ATP was initially not clear and was presumed to relate to substrate-level phosphorylation (as what happens in glycolysis).
Mitchell proposed that ATP could also be produced by chemiosmosis. He showed that ATP synthesis was coupled to an electrochemical proton gradient. This provided the basis for how oxidative phosphorylation led to ATP synthesis.
Chemiosmosis Model
Chemiosmosis is an energy-coupling mechanism employed by living organisms to produce ATP. In respiring cells, it is one of the major steps of cellular respiration. To further explain the process of chemiosmosis and describe how it is a part of cellular respiration, see the diagram below.
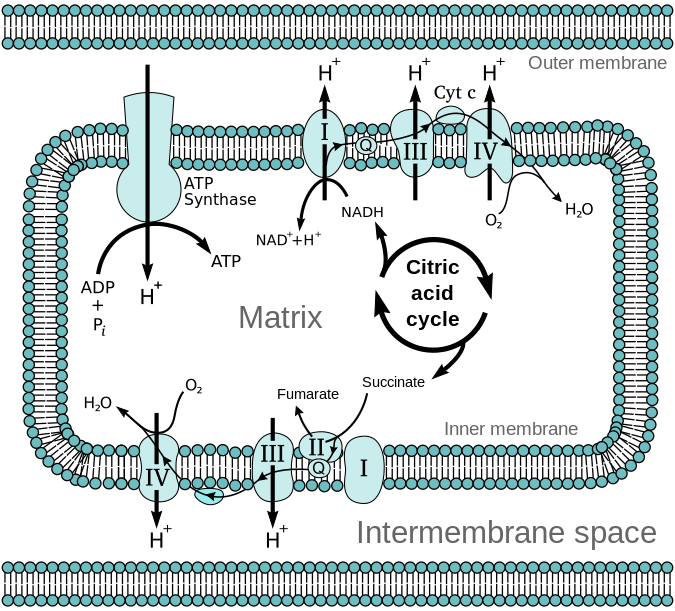
The figure above is a schematic diagram of the mitochondrion. It is regarded as the powerhouse of the cell because most ATPs are produced here. It is specialized for ATP synthesis. Take note that this organelle is a double-membraned structure. The mitochondrial membrane is made up of an outer membrane and an inner membrane. Both layers consist of lipid layers that prohibit the easy passage of ions.
In between the two membranes is the intermembrane space. The inner membrane forms many infoldings called cristae. The space within the inner membrane is called the mitochondrial matrix. The matrix is the location of the citric acid cycle, a cyclic metabolic reaction where food molecules are churned to generate energy-rich phosphate compounds. The pyruvate from glycolysis is converted into acetyl CoA that will enter the mitochondrion for complete oxidation and degradation into carbon dioxide.
For every pyruvate molecule, the citric acid cycle will generate one ATP via substrate phosphorylation. Most of the ATP will come from oxidative phosphorylation, which will take place at the mitochondrial membrane where the electron transport chain (ETC) and the enzyme ATP synthase are embedded.
Through redox reactions, most of the high-energy electrons are transferred to NAD+ and FAD to create NADH (and H+) and FADH2, respectively. These electron-carrying molecules will shuttle the electrons to the ETC for oxidative phosphorylation.
As the electrons are passed along the chain, every ETC member undergoes a redox reaction, accepting and donating electrons. The passing of electrons will reach the end — when the electrons are passed on to the final electron acceptor, the molecular oxygen.
The reaction subsequently forms water:
2 H+ + ½ O2 → H2O
ETC does not produce ATP. Instead, the ETC members pump H+ (protons) to the intermembrane space as electrons are passed along. (See the diagram above) As protons are pumped across, protons thereby accumulate on one side of the membrane. This creates a proton (H+) gradient. Researchers referred to it specifically as the proton-motive force. They define the term as the energy generated by the transfer of protons (or electrons) across an energy-transducing membrane.
The protons will move down to their gradient, i.e. from the intermembrane space to the matrix, through the channel of the ATP synthase. The hydrogen ion movement leads to ATP synthesis when the protons release the energy as they cross the ATP synthase. The energy causes the rotor and the rod of the enzyme to rotate. (Ref. 3) The enzyme is, then, activated to harness this force so as to build the high-energy bond between the ADP molecule and the inorganic phosphate (Pi) to produce an ATP molecule.
The reaction:
ADP + Pi → ATP
Function of Chemiosmosis
Chemiosmosis is about energy coupling. The relationship between chemiosmosis and ATP synthesis lies in the generation of a proton motive force. As explained earlier, cellular respiration employs chemiosmosis as the mechanism that drives ATP synthesis by oxidative phosphorylation. The electrons from the citric acid cycle (where pyruvate-turned-acetyl coenzyme A is broken down to carbon dioxide) are transferred to electron carriers to shuttle them to the ETC. The proton motive force that will develop from the protons accumulating on one side of the membrane during the energy transfer via a series of redox reactions in the ETC will, in turn, be used to build ATP from ADP and inorganic phosphate. Thus, without chemiosmosis, there will be no proton motive force for ATP synthase to use during ATP synthesis. As a result, there will be fewer ATP end products without chemiosmosis to incur the process. The same impact can be expected in photosynthesis where chemiosmosis is also a crucial step in ATP production.
Chemiosmosis Examples
Let’s take a look at some of the common examples where chemiosmosis occurs.
Chemiosmosis in chloroplasts
As described above, chemiosmosis takes place in the mitochondria of eukaryotes. But aside from the mitochondria, photosynthetic eukaryotes, such as plants, have another organelle where chemiosmosis takes place — the chloroplast.
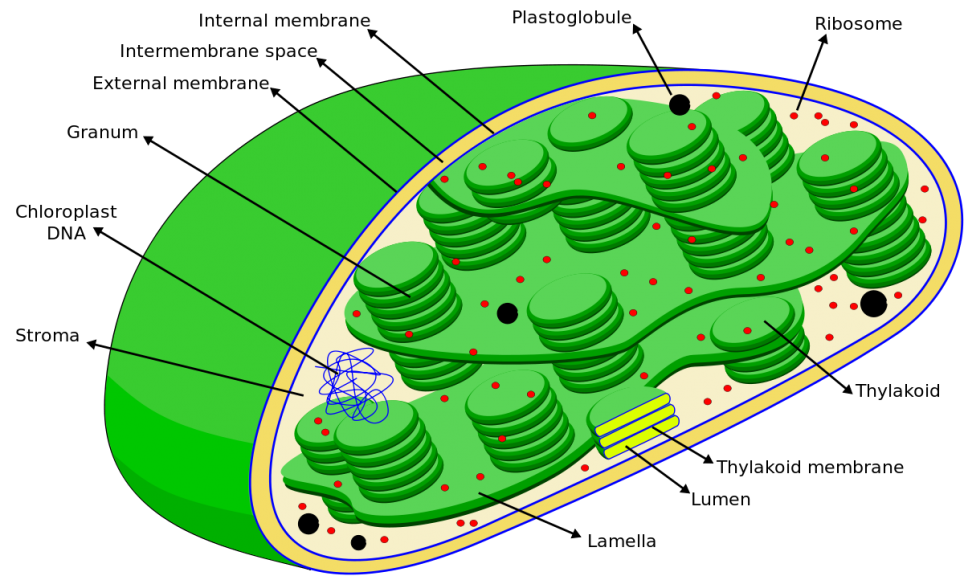
(Image credit: Vossman, CC BY-SA 4.0)
The chloroplast is the organelle involved primarily in photosynthesis. It has a thylakoid system that harvests light. Thus, it serves as the location for the light reactions (or light-dependent processes). The matrix of the chloroplast is referred to as the stroma. It is the thick fluid that contains enzymes, molecules, and other substrates involved in the dark reactions (or light-independent processes).
In chloroplast, chemiosmosis occurs in the thylakoid. This membrane system has its own transport chain and ATP synthases. One of the major differences between chemiosmosis in mitochondria and in chloroplasts is the source of energy. In mitochondria, the high-energy electrons are extracted from the food molecule (from redox reaction) whereas in chloroplast the source is from the photons captured from the light source. The proton (H+) gradient forms from the H+ ions accumulating in the thylakoid compartment (i.e. the space inside the thylakoid).
H+ ions can come from (1) the splitting of water during the light reactions, (2) from the protons that are translocated across the thylakoid membrane as electrons are passed along the transport chain, and (3) from stromal H+ ions picked up by NADP+. As H+ ions are greater in number inside the thylakoid compartment (lumen), they will diffuse to the stroma by crossing the ATP synthases embedded in the thylakoid membrane.
Chemiosmosis in prokaryotic cells
In prokaryotes such as bacteria and archaea, chemiosmosis occurs in the cell membrane since these organisms lack mitochondria and chloroplasts.
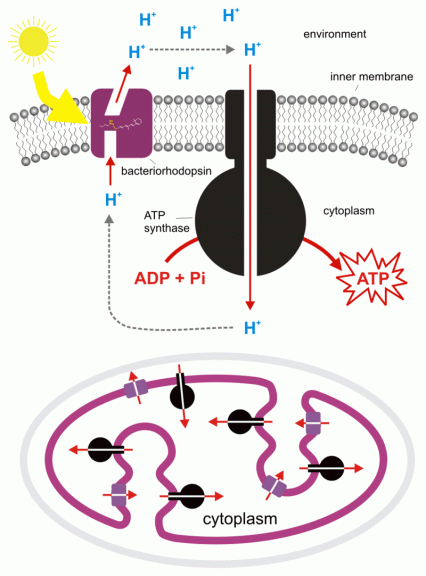
The hydrogen ions (protons) move across the biological membrane via the ATP synthase (a transport protein) when a proton gradient forms on the other side of the membrane. The proton gradient forms when the hydrogen ions accumulate as they are forcibly moved to the other side during the electron transport and redox reactions. As more hydrogen ions are on the other side they will move back to the cell move by crossing the membrane through the ATP synthase. As they flow through, energy is released and used to convert ADP to ATP through phosphorylation.
Chemiosmosis vs. Oxidative Phosphorylation
Oxidative phosphorylation is a metabolic pathway that generates ATP from the energy produced through a series of redox reactions in the ETC. Thus, it is also called electron transport-linked phosphorylation. It is an aerobic process since molecular oxygen is the final electron acceptor. This makes it different from the other form, i.e. substrate-level phosphorylation where ATP is generated directly from an intermediate substrate. Oxidative phosphorylation, by contrast, is an indirect way of synthesizing ATP. It is coupled with chemiosmosis where protons are moved across the membrane.
Chemiosmosis is the mechanism used by oxidative phosphorylation to directly make ATP. However, ATP synthase will not be able to do so without the proton motive force that results from the ETC that moves protons (H+) to the other side of the membrane as the electrons are passed along the chain.
NOTE IT!
How Chemiosmosis Powered the Rise of Complex Life
Chemiosmosis played an essential role in the evolution of complex life forms, especially in the emergence of aerobic organisms. Here are the possible ways by which chemiosmosis contributed to the development of complex living things, like the ones capable of utilizing oxygen.”
- Chemiosmosis enabled living things to generate ATP stably and in amounts sufficient to support more complex and energy-demanding biological processes
- Chemiosmosis provides a more efficient method of generating ATP than many other methods (e.g., fermentation, photophosphorylation, substrate-level phosphorylation). Chemiosmosis takes advantage of first, a proton-motive force containing potential energy that can be harnessed for ATP synthesis and second, the activity of ATP synthase enzymes that rapidly convert the proton motive force into ATP.
- With chemiosmosis, ecological niches expanded and diversified, enabling organisms to explore new habitats, from aquatic to terrestrial, and exploit diverse nutritive substances, particularly oxygen.
Chemiosmosis became instrumental in what is known as the “oxygen revolution” on Earth. Oxygen released by the early photosynthetic prokaryotes in a previously anaerobic atmosphere was eventually harnessed by other organisms. Because oxygen has a high affinity for electrons, it served efficiently as the terminal electron acceptor in the ETC. The transfer of electrons in the ETC has been so efficacious that it produced a strong proton motive force that meant more ATP yield. Chemiosmosis coupled with ETC made it a cornerstone of cellular energy production, which in turn, empowered the evolution of aerobic life — from the relatively simpler prokaryotes to the more complex eukaryotes, from the single-celled to multicellular life forms.
Try to answer the quiz below to check what you have learned so far about chemiosmosis.
References
- Mitchell, P. (1961). “Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemi-Osmotic type of Mechanism”. Nature. 191 (4784): 144–148. https://doi.org/10.1038%2F191144a0
- Berg, J. M., Tymoczko, J. L., & Lubert Stryer. (2013). A Proton Gradient Across the Thylakoid Membrane Drives ATP Synthesis. Nih.Gov; W H Freeman. https://www.ncbi.nlm.nih.gov/books/NBK22519/
- 18.3D: Electron Transport Chain and Chemisomosis. (2016, March 22). Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Microbiology/Book%3A_Microbiology_(Kaiser)/Unit_7%3A_Microbial_Genetics_and_Microbial_Metabolism/18%3A_Microbial_Metabolism/18.3%3A_Aerobic_Respiration/18.3D%3A_Electron_Transport_Chain_and_Chemisomosis
©BiologyOnline. Content provided and moderated by BiologyOnline Editors.