Cohesion
n., koʊˈhiʒən
The act, state or process of sticking together
Table of Contents
Cohesion Definition
What is cohesion? Cohesion, in science definition, refers to the state of cohering or sticking together of alike entities. Cohesion can be observed naturally in certain molecules, such as water. What causes them to cohere or stick together is the intermolecular force that holds them together. Nevertheless, those that are attracted to other molecules or to a different substance do not exhibit cohesion. Rather, this phenomenon is called adhesion.
The meaning of cohesion is applicable to biology as well. It refers to the process, act, or state in which alike molecules or body parts bind or stay close together. In botany, for instance, the term may refer to the fusion of plant parts, such as in syncarpy (the fusion of carpels of a pistil).
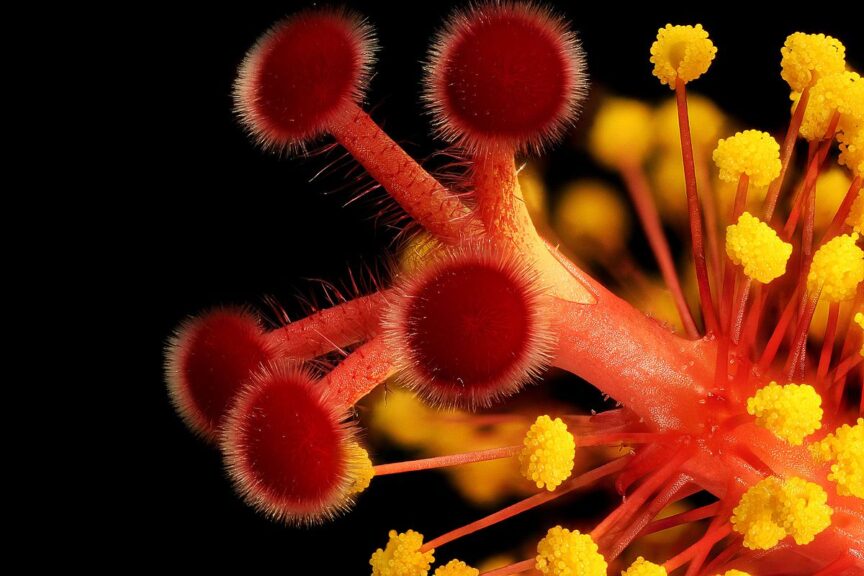
An example of a syncarpous gynoecium wherein the five carpels in the middle are in cohesion. The pollen-bearing anthers are found below the fused carpels. Credit: Benjamin Smith (CC BY-2.0)
Cohesion is the act, state, or process of sticking together of alike molecules or entities. An example is water molecules. The tendency of water molecules to stick together is referred to as cohesion and they are held together by a cohesive force such as an intermolecular hydrogen bond.
Etymology: from Latin cohaesiō, from cohaereō, meaning “to cling” “to stick together”. Synonyms: cohesive force; cohesive attraction. Compare: adhesion
Cohesion Examples and Biological Importance
Cohesion of water molecules
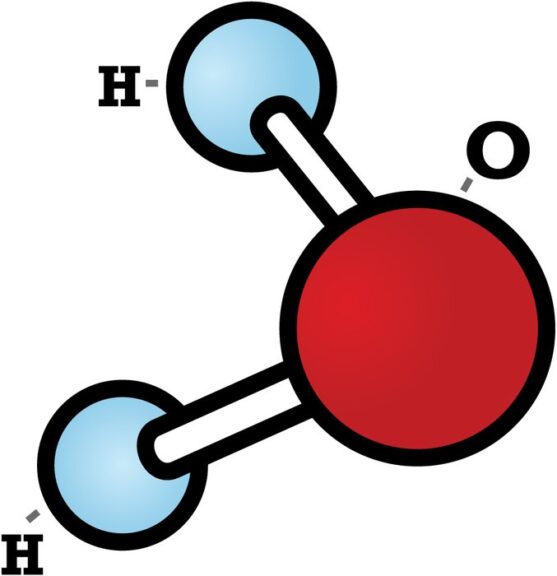
Water is an example of a substance exhibiting cohesion. Water consists of dihydrogen monoxide (HOH) molecules, meaning two hydrogens and one oxygen. Take a look at the schematic diagram of the chemical structure of a water molecule. The molecule exhibits polarity, which means the state of having two opposite charges. The oxygen has a slightly negative charge resulting in a partially negative pole whereas its hydrogens have a slightly positive charge resulting in a partially positive pole. Polarity makes water molecules stick or attract one another.
Water molecules are held together by a cohesive force. This force is a weak or transient type of chemical bond referred to as an intermolecular hydrogen bond. It forms between the hydrogen of one HOH and the hydrogen of another HOH. As a result, they form a water drop as they cohere. (Ref. 1)
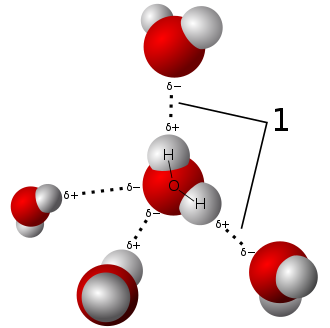
Apart from cohesion, water also exhibits adhesion. While cohesion pertains to the attraction between similar molecules, adhesion refers to the attraction of dissimilar molecules. So while cohesion causes water to form drops, adhesion keeps the drop on a surface, as seen on the surface of leaves or flowers.
If you will let water flow gradually through a dropper, you will notice that it flows in a series of drops rather than continuously. The drop also assumes a shape that is somewhat spherical (gravity causes the drop to lose its supposedly perfect sphere shape). (Ref. 2) This is due to surface tension. Have you noticed when you fill the glass with water up to the rim it forms a dome-like shape on top? That’s surface tension.
Surface tension, by definition, refers to the attractive force that is exerted by the molecules below the surface molecules causing the liquid to assume a shape with the least surface area. (Ref. 3) This term is used particularly when the liquid surface is in contact with the gas, for example, air. (Ref. 2) It makes the surface of the water resist rupture even while under tension or stress. What causes surface tension in water is attributed essentially to cohesion.

Notice the water drop on the leaf surface in the photo. The spherical shape of water is attributed to the surface tension of water. The surface tension is due to cohesion (water molecules attracting each other). In this example, the cohesive force (force between water molecules) appears stronger than the adhesive force (force between a water molecule and other molecules, such as the molecules of the air and leaf surface).
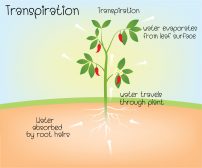
Water molecules have a greater attraction to each other than with molecules in the air. Thus, they tend to exhibit surface tension. This is important to plants as cohesion and high surface tension tend to slow down water loss — i.e. the water that leaves through leaf stomata.

Surface tension is also what causes certain insects to stay still above the water or to walk through it. In the photo below, notice how the water strider is able to stand still without sinking its legs below the water surface.
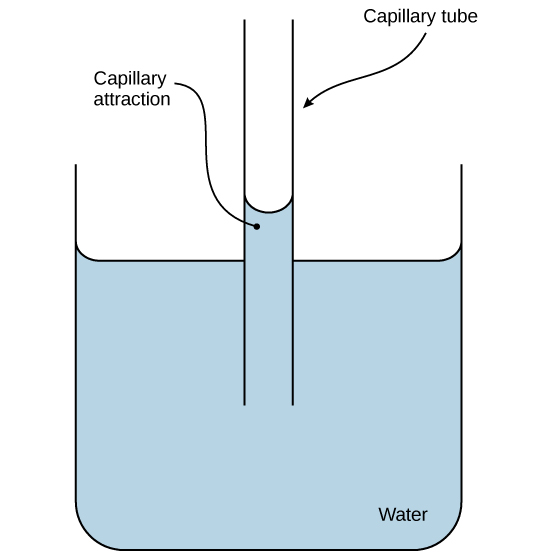
Surface tension, cohesion, and adhesion are the essential factors that enable capillarity. This is important, especially in vascular plants. Water can move up a narrow tube against gravity due to the capillary action. Surface tension pulls the molecules of the liquid inward from the surface resulting in the least surface area possible. Then, cohesion is responsible for the water molecules to stick together. Adhesion helps the water molecule to bind to the walls of the xylem tissues of a plant. Thus, water can ascent from the root upward through the xylem.
Cohesion of biomolecules
Cohesion is not only a physicochemical phenomenon. It also occurs biologically as exhibited by biomolecules, such as DNA. If you will remember in cell division, such as mitosis and meiosis, sister chromatids cohere until they separate during the anaphase. This cohesion event is mediated by various protein complexes that are collectively called cohesins. Below is a diagram depicting the four protein subunits of cohesin: SMC3, SMC1, SCC1, and SCC3. The cohesins hold the sister chromatids after the DNA is duplicated in preparation for cell division. It ensures that the sister chromatids will be kept connected with each other up until they reach the equatorial region of the dividing cell. It is the loss of cohesion between the sister chromatids that complete separation during the anaphase is enabled. Without cohesins, proper segregation may not be guaranteed. (Ref. 4) Both mitosis and meiosis utilize cohesion to keep the sister chromatids together and cohesion is established prior to mitosis and meiosis. (Ref. 4)

How is Cohesion Vital to Life
Cohesion, as we have noted earlier, is an act or process by which entities join or stick together. This is important as it serves a fundamental role in sustaining life. How? Here are a few of the many ways:
- Water transport in plants: Because water molecules tend to stick together, water can form a continuous column, especially as they travel through the xylem vessels of plants. Cohesion, though, is not the only way by which plants enable water transport across the xylem vessels. Another force comes from the ability of water molecules to stick to the walls of the xylem (called adhesion). The combined forces of cohesion and adhesion facilitate the upward transport of water — from roots to leaves. Water is crucial in different biological processes, such as photosynthesis.
- Blood flow in animals: Cohesion helps in the efficient transport and flow of blood through veins and arteries. For instance, water molecules stick togethe, helping stabilize the blood and preventing it from fragmenting. Another way by which cohesion is crucial in this sense is its effect on blood viscosity. The cohesive forces that act among blood components help regulate blood viscosity or thickness. Balance in blood viscosity — i.e., not too thick and not too thin –– is essential as blood that is too viscous or dense can lead to increased resistance and strain on the blood vessels and the heart. Blood that is less dense or thin could mean inefficiency in transporting nutrients and oxygen. Cohesion, therefore, helps in keeping this delicate balance in blood.
Take the quiz about cohesion!
References
- Water Drops: Cohesion and Adhesion of Water. (2020). Appstate.Edu. http://www.appstate.edu/~goodmanjm/rcoe/asuscienceed/background/waterdrops/waterdrops.html
- What is Surface Tension? Definition and Experiments. (2020). ThoughtCo. https://www.thoughtco.com/surface-tension-definition-and-experiments-2699204
- Definition of SURFACE TENSION. (2020). Merriam-Webster.Com. https://www.merriam-webster.com/dictionary/surface%20tension#:~:text=%3A%20the%20attractive%20force%20exerted%20upon,having%20the%20least%20surface%20area
- Brooker, A. S., & Berkowitz, K. M. (2014). The Roles of Cohesins in Mitosis, Meiosis, and Human Health and Disease. Methods in Molecular Biology, 229–266. https://doi.org/10.1007/978-1-4939-0888-2_11
©BiologyOnline. Content provided and moderated by BiologyOnline Editors.