Cross linking
n., [ˈkrɔsˌlɪŋkɪŋ]
Definition: in biology, the joining adjacent biological structures with cross-link
Table of Contents
Cross-linking Definition
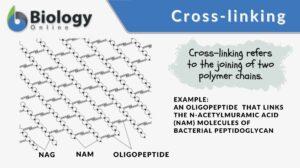
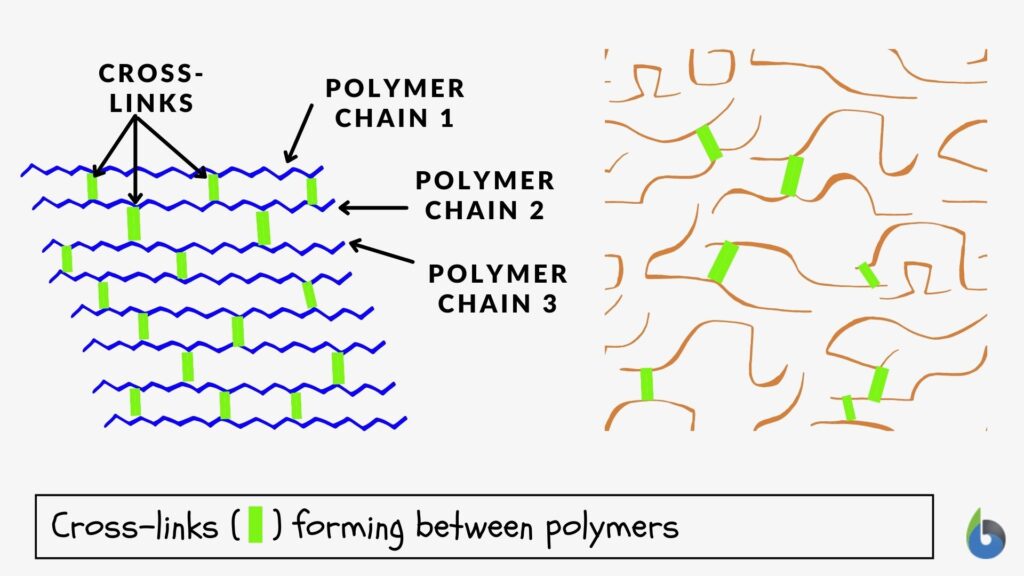
Cross-linking, in general, means the forming of cross-links between the joining structures. In biology, a cross-link is a link or a “bridge” between two biological structures. Take a look at the schematic diagram below. The structures (polymers) are joined together by cross-links.
Cross-linking is the linking between polymers via a chemical bond(s). In the biological sense, it refers to the joining of biological structures in cross-links, such as the linking of two strands of DNA by covalent bonds when exposed to x-rays and the cross-links forming in the peptidoglycan of the bacterial cell wall.
Cross-Linking Examples
In biochemistry, a cross-link is essentially a chemical bond that forms between two molecules (intermolecular) or within the molecule (intramolecular). The network formed by the cross-linking will have features or properties that may depend on the degree of cross-linking. In most cases, the more cross-links, the stronger and more resistant it is to dissolution.
Corneal Collagen Cross-linking
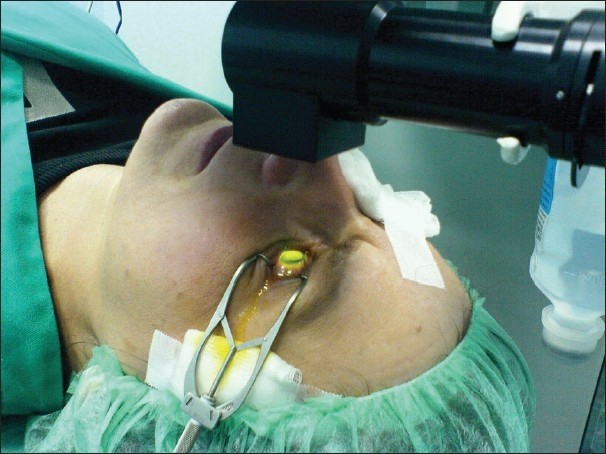
This molecular bonding is, in fact, the working principle behind corneal collagen cross-linking (CXL). This procedure is applied to treat keratoconus, a progressive eye disease wherein the eye eventually becomes conical in shape rather than the normal round shape. The procedure makes use of UVA (ultraviolet-A) light to activate the riboflavin (Vit B2), which was initially administered to the patient via eye drops on the surface of the cornea. Through the UVA light and riboflavin (photosensitizer), cross-links are formed in the corneal tissue. More covalent bonds form between and within the fibrillar component of the corneal tissue are produced by the treatment. The result is a stronger cornea. This then stops or reduces the progression of keratoconus. (Jankov Ii et al., 2010)
Abnormal linking of DNA strands
The abnormal linking of two strands of DNA by covalent bonds (as opposed to the normal hydrogen bonds between base pairs) is another example of cross-linking. It can occur by exposure to x-rays. Such linking is a type of damage to the DNA molecule and must be repaired before the DNA can replicate and function properly again.
The cell wall of eubacteria
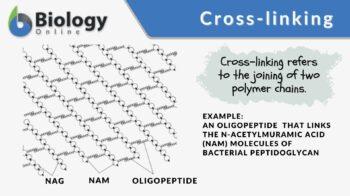
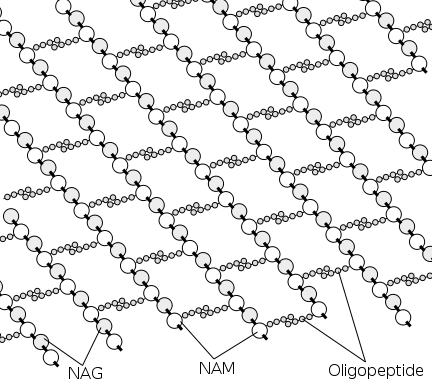
Peptidoglycan (or murein), the crystal lattice structure that forms the cell wall of a bacterial cell, is formed by chains of alternating N-acetylglucosamine and N-acetylmuramic acid. The chains are connected together by cross-links (short peptide chains called oligopeptide) between N-acetylmuramic acid molecules.
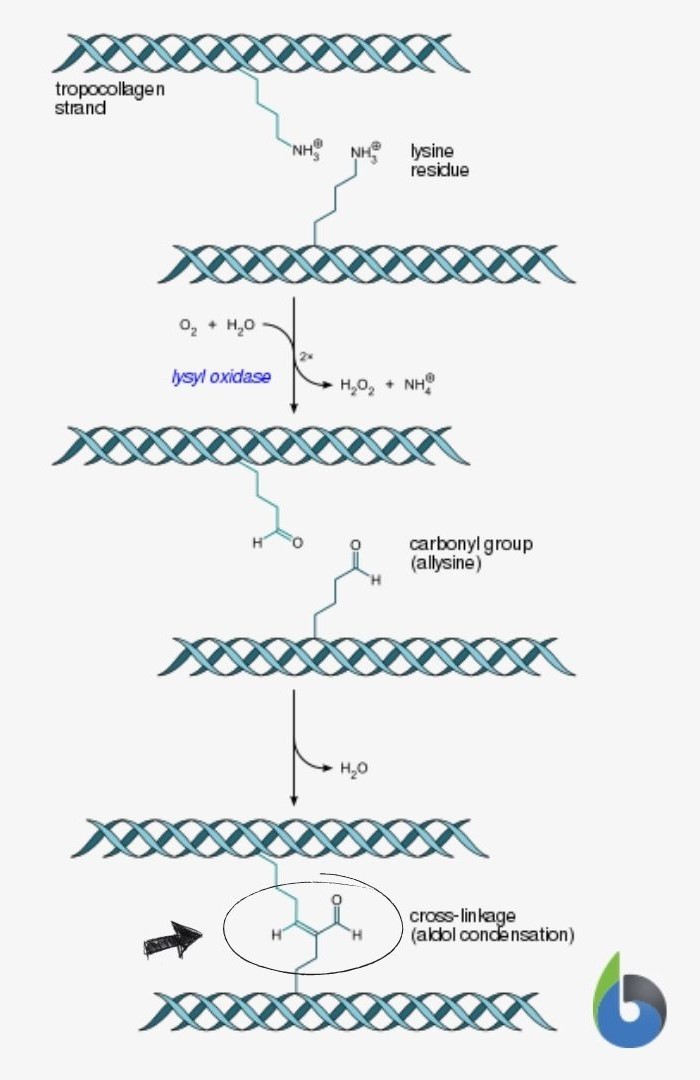
Cross-linking in the biosynthesis of collagen
Collagen is one of the main fibrillar components of the extracellular matrix (ECM). How does the body produce collagen? Let’s trace the main steps of the biosynthesis of collagen type I below. (News-Medical, 2011)
- First, the cell produces a procollagen (a triple helical structure made up of two alpha-1 chains and one alpha-2 chain), then secretes it by exocytosis via a secretory vesicle.
- When outside the cell, the enzymes “collagen peptidases” remove the “loose ends” of the procollagen, forming tropocollagen. Tropocollagen is the structural unit of collagen fiber.
- Tropocollagen molecules gather, forming collagen fibrils, through cross-linking. The covalent bond forms between hydroxylysine and lysine residues via the enzyme, lysyl oxidase.
- The aggregation of collagen fibrils forms the collagen fiber. The collagen may then be attached to the cell membrane via fibronectin and integrin.
Biological Role of Cross-linking
The presence of cross-links makes the polymer chains be bound together. The extent of movement will be lesser. As they are linked together, they are more rigid, stronger, and tougher, and therefore can be great in providing mechanical support to a biological structure. In bacteria, the thickness of the polypeptide layer of the bacterial cell wall determines if the bacteria are Gram-positive or Gram-negative. Gram-positive bacteria have significantly thicker peptidoglycan. They are also more susceptible to antibacterial agents, particularly, penicillin and β-lactam antibiotics, that act by destroying the cross-links in the peptidoglycan of Gram-positive bacteria, making the latter relatively fragile and more susceptible.
Watch this vid on bacterial polypeptide:
In human tissues, while cross-linking is a natural step in collagen formation and as such fosters stability and structural strength, experts believe that cross-linking could impair the function of tissues and organs with age. Extracellular glycation cross-linking could tighten up the ECM and reduce the elasticity of blood vessels and the urinary bladder. (Furber, 2006) Cross-links, if in excess or non-specific (via glycation), may therefore lead to tissue or organ stiffness. Over time, more and more proteins and other polymers are “attached” to one another via cross-links, thus, making the tissue stiffer and stiffer with age.
Take the Biology Quiz on cross-linking!
References
- Arora, B., Tandon, R., Attri, P., & Bhatia, R. (2017). Chemical Crosslinking: Role in Protein and Peptide Science. Current Protein & Peptide Science, 18(9). https://doi.org/10.2174/1389203717666160724202806
- Furber, J. D. (2006). Extracellular Glycation Crosslinks: Prospects for Removal. Rejuvenation Research, 9(2), 274–278. https://doi.org/10.1089/rej.2006.9.274
- Jankov Ii, M. R., Jovanovic, V., Nikolic, L., Lake, J. C., Kymionis, G., & Coskunseven, E. (2010). Corneal collagen cross-linking. Middle East African Journal of Ophthalmology, 17(1), 21–27. https://doi.org/10.4103/0974-9233.61213News-Medical. (2011, January 18). Collagen Synthesis.
- News-Medical.net. https://www.news-medical.net/health/Collagen-Synthesis.aspx
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.