Cytokinesis
n., plural: cytokineses
[ˌsaɪtəʊkɪˈniːsɪs]
Definition: The division of the cytoplasm
Table of Contents
The cell cycle of eukaryotes is a cyclical series of biological events that certain asexual cells go through. The cell cycle is comprised of these fundamental events: (1) resting phase (Gap 0), (2) interphase (Gap 1, S phase, Gap 2), and (3) cell division (i.e. mitotic phase and cytokinesis). In essence, the cell may enter a quiescent stage called the resting phase or it may go through the rest of the phases of the cell cycle. If the cell is committed to undergoing mitosis, it will replicate its DNA during interphase and enters cell division.
Cytokinesis Definition
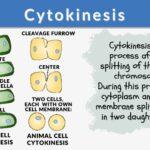
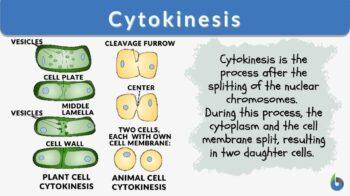
Cytokinesis is the process following the division of the nucleus, the cytoplasm and plasma membrane are divided, resulting in two cells, each with its own nucleus and cytoplasm surrounded by a plasma membrane. It occurs in both plant cells and animal cells.
Cells are the building blocks of most organisms. Cells undergo all kinds of processes and functions. These include processes like metabolism and active transport. In order to carry out these processes, cells must multiply. They usually do this through the process of cytokinesis. What is cytokinesis? What happens during cytokinesis?
To define cytokinesis, one must think of cytokinesis in biology. Cytokinesis is the cell cycle stage in which the cell must split its chromosomes and cytoplasm, producing two daughter cells. Cytokinesis is the division of the cytoplasm. The cytoplasm of the cell is being divided in order to obtain two new daughter cells.
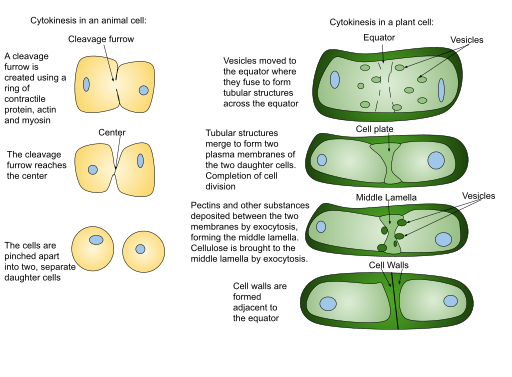
How does cytokinesis occur? Cytokinesis occurs in mammalian cell division when a contractile ring of microfilaments generates a cleavage furrow that pinches the cell membrane in two. A cell plate is built in plant cells to divide the cell in half. A cytokinesis diagram can be viewed below in figure 1.
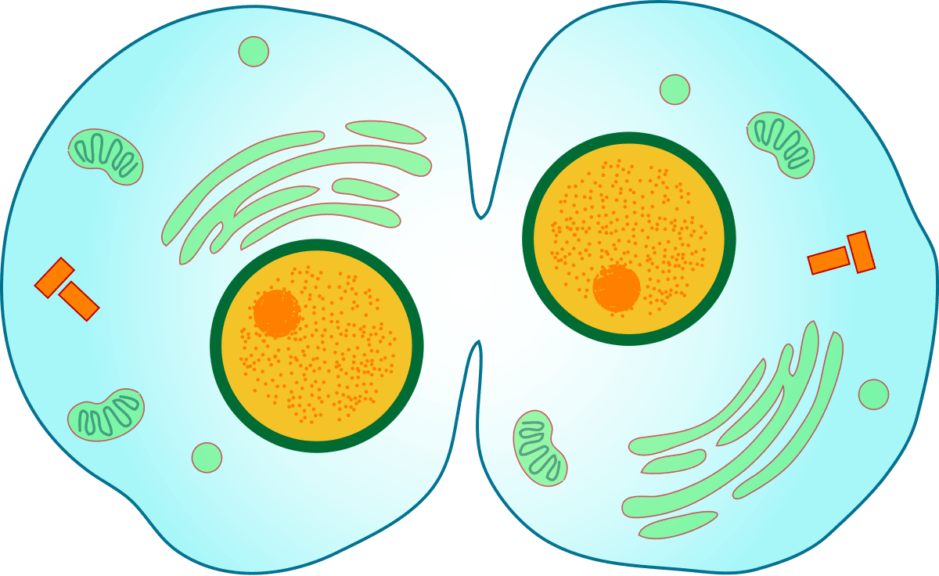
When does cytokinesis occur? Is cytokinesis part of mitosis? Cytokinesis starts in anaphase and concludes in telophase, finishing as the next interphase begins. These are all steps in the mitosis of an animal cell or plant cell mitosis.
There are two types of cell division — mitosis and meiosis, and cytokinesis can occur in both. The abrupt emergence of a pucker, or cleavage furrow, on the cell surface, is the first apparent modification during cytokinesis in animal cells.
Particular activities necessitate departures from the symmetrical cytokinesis process; for example, in mammal oogenesis, the ovum consumes practically all of the cytoplasm and organelles. This leaves relatively little for the ensuing polar bodies, which die without function in most species but take on certain particular roles in others. Another kind of mitosis occurs in organs such as the liver and skeletal muscle; it lacks cytokinesis and produces multinucleate cells.
How is cytokinesis different in plants and animals? Plant cytokinesis varies from animal cytokinesis in part because cytokinesis in plant cells happens through the stiffer cell walls. What is a cell plate? Instead of a cleavage furrow emerging between plant daughter cells, a dividing structure called the cell plate forms in the cytoplasm and expands into a new, doubled cell wall between plant daughter cells. The cell is then divided into two cells typically called the daughter cells.
Cytokinesis is similar to the prokaryotic process of binary fission, but the processes differ due to changes in bacterial and eukaryotic cell shapes and roles. In contrast to the linear, typically numerous, chromosomes of eukaryotes (chromosomes in animal cells), a bacterial cell contains a circular chromosome (a single chromosome in the form of a closed-loop).
As a result, bacteria do not form mitotic spindles during cell division. Furthermore, duplication of prokaryotic DNA occurs before chromosomal separation; in mitosis, duplication occurs during the interphase before mitosis begins, however, the daughter chromatids do not split entirely before anaphase.
Spermatogenesis is an example of a process with cytokinesis. It is a cell division process in males in which freshly generated sperm cells are equal in size and content. Oogenesis is an asymmetrical cytokinesis example; in females, it creates a big egg cell with three polar bodies.
Mitosis cytokinesis
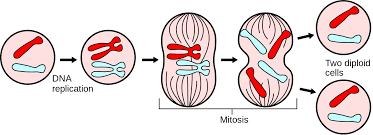
Mitosis is the process by which a parent cell divides to produce two identical daughter cells in eukaryotic cells. Mitosis is the separation of the duplicated genetic material contained in the nucleus during cell division. Prophase, as well as prometaphase and metaphase, anaphase, and finally telophase, are the phases of mitosis. All the cellular Egbert is sent towards cell division during mitosis. There is no cell growth of the cell.
During prophase, replicated chromosomal pairs compress and compact themselves. Sister chromatids are pairs of replicated chromosomes that stay connected at a central place known as the centromere. Long proteins called microtubules create a big structure called the mitotic spindle on either side or pole, of the cell.
What happens during metaphase? The nuclear envelope that encloses the nucleus breaks down during prometaphase, and the nucleus is no longer isolated from the cytoplasm. Kinetochores are protein structures that occur around the centromere. The mitotic spindle grows from the poles and joins the kinetochores. During metaphase, the microtubules drag the sister chromatids back and forth until they align in a plane at the cell’s center called the equatorial plane.
During anaphase, the sister chromatids are split at their centromeres simultaneously. The spindle then pulls the divided chromosomes to opposing directions or poles of the cell. The anaphase cell guarantees that each daughter cell has the same set of chromosomes.
When is cytokinesis complete? Finally, a nuclear membrane grows around each set of chromosomes during telophase animal cells to isolate the nuclear DNA from the cytoplasm. The chromosomes start to uncoil, becoming more scattered and less compact. Along with the telophase cell, the cell goes through a second process termed cytokinesis, which separates the mother cell’s cytoplasm into two daughter cells. These are the products of mitosis and cytokinesis.
Meiosis Cytokinesis
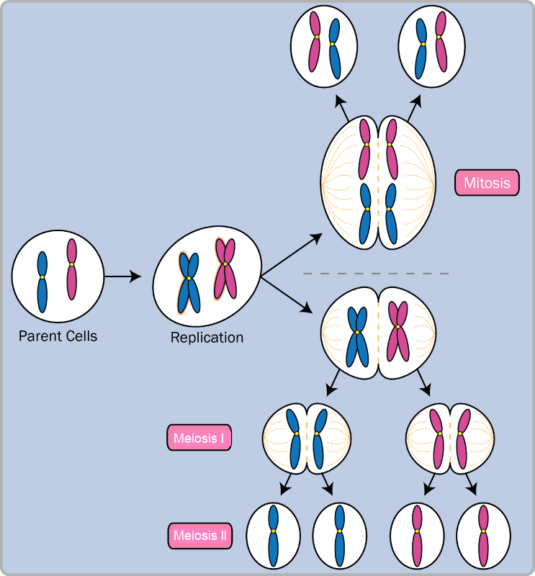
Meiosis, also known as reduction division, is the division of a germ cell that involves two nucleolus fissions and produces four gametes, or sex cells, each with half the number of chromosomes as the original cell.
Meiosis is a process that occurs in organisms that reproduce sexually. Such organisms contain a diploid (double) set of chromosomes in the nucleus of each cell, consisting of two haploid sets (one inherited from each parent). In humans, the two sets occur as homologous chromosomes (including the sex chromosome pair XX chromosomes but not XY chromosomes).
Each chromosome in the diploid germ cell has duplicated and hence consists of a joined pair of duplicate chromatids before meiosis. The contraction of the chromosomes in the nucleus of the diploid cell initiates meiosis. Paternal and maternal chromosomes join up along the cell’s midline. Each chromosomal pair is made up of four chromatids. This is known as a tetrad or bivalent. At this stage, homologous chromosomes exchange genetic material by crossing over.
The homologous pairs are subsequently dragged to opposing ends of the cell, which pinches in half to generate two daughter cells. This first meiotic division (cytokinesis 1) produces a haploid pair of chromosomes in each daughter cell. This first meiotic division produces a haploid pair of chromosomes in each daughter cell. At this time, the chromosomes are still made up of double chromatids. This is the end of telophase 1. One division brings the cell to the end of the process of mitosis.
Each haploid daughter cell divides during the second meiotic division also known as cytokinesis 2. This division results in no additional reduction in chromosomal number because it entails the separation of each chromatid pair into two chromosomes that are drawn to opposing ends of the daughter cells.
Each daughter cell then splits in half, yielding four distinct haploid gametes. When two gametes mate, each contributes its haploid set of chromosomes to the new person, restoring the diploid number.
Watch this vid about cytokinesis in plant cells and animal cells:
Cytokinesis is the division of the cytoplasm and the plasma membrane following the division of the nucleus resulting in two cells, each having its own nucleus and cytoplasm surrounded by a plasma membrane. Cell division is comprised of the mitotic phase and cytokinesis. During the mitotic phase, the cell will give rise to two daughter nuclei, each with identical genetic content. Cytokinesis is that phase in the cell cycle wherein other cellular components are separated resulting in the production of two daughter cells from the original (parent) cell. In animal cells (and in some plant cells) it is marked by a constriction of the cell membrane called the cleavage furrow. In most plant cells it happens when an expanding cell plate forms across the center of the cytoplasm called the phragmoplast. The separation of cellular components may also occur in meiosis, particularly following telophase II of the second meiotic division.
Etymology: Greek kyto-, kýtos (container, receptacle, body) + Greek kīnēsis (movement). Compare: karyokinesis. See also: mitosis, cell cycle.
Animal Cell
In animal cell, cytokinesis occurs shortly after the commencement of sister chromatid separation during mitosis’s anaphase. The steps are as follows: anaphase spindle rearrangement, division plane selection, actin-myosin ring construction and contraction, and abscission. The precise temporal coordination of the aforementioned individual steps by molecular signaling pathways ensures faithful partitioning of the genome to developing daughter cells.
Anaphase spindle reorganization
Animal cell cytokinesis begins with microtubule stabilization and restructuring of the mitotic spindle to create the central spindle. When non-kinetochore microtubule fibers connect between the spindle poles, the central spindle (or spindle midzone) develops.
The central spindle is required for cytokinesis in a variety of species, including H. sapiens, D. melanogaster, and C. elegans, albeit the specific phenotype associated with its absence, varies from species to species. This could be, for instance, in certain cells in the Drosophila. These are not able to form cleavage furrows without first having a central spindle. On the other hand, both C. elegans embryos and human tissue culture cells contain a cleavage furrow that forms and ingress. These then regress even before cytokinesis is complete. The decrease in CDK1 activity during anaphase causes the process of mitotic spindle remodeling and central spindle formation.
Dephosphorylation of inhibitory sites on several central spindle components occurs as CDK1 activity declines during the metaphase-anaphase transition. To begin with, removing CDK1 phosphorylation from a component of the CPC (chromosomal passenger complex) permits its translocation from the centromeres to the central spindle during metaphase.
CPC, in addition to being a structural component of the central spindle, participates in the phosphoregulation of other central spindle components such as PRC1 (microtubule-bundling protein necessary for cytokinesis 1) and MKLP1 (a kinesin motor protein). PRC1, which was previously blocked by CDK1-mediated phosphorylation, may now form a homodimer that preferentially binds to the interface of antiparallel microtubules, promoting the spatial organization of the central spindle’s microtubules.
The centralspindlin complex is formed by MKLP1 and the Rho-family GTPase activating protein CYK-4 (also known as MgcRacGAP). Higher-order clusters of centralspindlin bind to the central spindle. The phosphorylation of MLKP1 by Aurora B, a component of CPC, promotes the development of centralspindlin clusters. In short, the self-assembly of the central spindle is triggered by the phosphoregulation of several central spindle components caused by the reduction of CDK1 activity at the metaphase-anaphase transition, either directly or indirectly.
The central spindle may play several roles in cytokinesis, including controlling cleavage furrow location, delivering membrane vesicles to the cleavage furrow, and forming the midbody structure necessary for the last phases of division.
Division plane specification
The second stage of animal cell cytokinesis involves the selection of division planes and the creation of cytokinetic furrows. To prevent chromosomal loss, the division plane between the two masses of separated chromosomes must be precisely positioned.
Meanwhile, the method by which the spindle decides the division plane in animal cells is one of cytokinesis’s most persistent mysteries and a source of great controversy. Furrow induction can be explained by one of three ideas. The astral stimulation concept proposes that astral microtubules from the spindle poles transport a furrow-inducing signal to the cell cortex, where impulses from two poles are somehow concentrated into a ring at the spindle.
The central spindle theory proposes that the cleavage furrow is caused by a positive stimulus that originates in the central spindle equator. The central spindle may help to define the division plane by increasing the concentration and activity of the small GTPase RhoA in the equatorial cortex.
The astral relaxation idea is a third possibility. It is hypothesized that active actin-myosin bundles are dispersed throughout the cell cortex and that inhibiting their contraction at the spindle poles leads to a gradient of contractile activity that is greatest the halfway between poles. In other words, astral microtubules produce a negative signal that causes cortical relaxation at the poles to rise.
In C. elegans embryos, genetic and laser-micromanipulation investigations revealed that the spindle delivers two redundant signals to the cell cortex, one from the central spindle and the other from the spindle aster, implying the participation of numerous processes in the placement of the cleavage furrow. The dominance of one signal over another varies between cell types and species. Furthermore, a large number of signals with partial redundancy may be necessary to make the system more resilient and enhance spatial accuracy.
Actin-myosin ring assembly and contraction
The actin-myosin contractile ring drives the cleavage process at the cytokinesis furrow, during which the cell membrane and wall expand inward, finally pinching the mother cell in half. The filamentous protein actin and the motor protein myosin II are essential components of this ring. The contractile ring forms at the cell cortex equatorially (in the middle of the cell adjacent to the cell membrane).
In animal cells, the Rho protein family (RhoA protein in mammalian cells) is a critical regulator of contractile ring formation and contraction. Two key effectors of the RhoA pathway enhance actin-myosin ring construction.
First, by activating Diaphanous-related formins, RhoA promotes the formation of unbranched actin filaments. This limited synthesis of new actin filaments is critical for the development of contractile rings.
Profilin, a protein that binds to actin monomers and helps load them onto the filament end, is also required in the actin filament production process. Second, RhoA stimulates myosin II activation through the kinase ROCK, which directly activates myosin II by phosphorylating the myosin light chain and inhibits myosin phosphatase by phosphorylating the phosphatase-targeting component MYPT. The contractile ring comprises the scaffolding protein anillin in addition to actin and myosin II.
Anillin binds to actin, myosin, RhoA, and CYK-4, connecting the equatorial cortex to central spindle signals. It also helps to connect the actin-myosin ring to the plasma membrane. Anillin also creates contractile forces by correcting thermal variations. Another protein, septin, has been proposed to act as a structural scaffold for the organization of the cytokinesis machinery. Following its formation, the actin-myosin ring contracts, allowing the associated plasma membrane to enter and divide the cytoplasm into two domains of developing sister cells.
Myosin II, the motor protein, generates force for contractile processes by moving along actin. Myosin II moves along actin filaments, restricting the cell membrane to generate a cleavage furrow, using the free energy released when ATP is digested. With continued hydrolysis, this cleavage furrow ingresses (moves inwards), a stunning phenomenon evident under a light microscope.
Abscission
The cytokinetic furrow ingresses until a midbody structure (made of electron-dense, proteinaceous material) is created, at which point the actin-myosin ring has a diameter of around 1–2 m. Most animal cell types stay united by an intercellular cytokinetic bridge for several hours until being separated by the last phase of cytokinesis, an actin-independent process called abscission.
The abscission process physically divides the midbody into two halves. Abscission occurs by the loss of cytoskeletal structures from the cytokinetic bridge, constriction of the cell cortex, and fission of the plasma membrane. Dense bundles of antiparallel microtubules derived from the central spindle fill the intercellular bridge. The midbody of these microtubules is assumed to constitute a targeting platform for the abscission machinery.
Spastin, a microtubule severing protein, is mostly responsible for microtubule bundle deconstruction inside the intercellular bridge. The loss of the underlying cytoskeletal structures is also required for complete cortical constriction. The PKC–14-3-3 complex, which inactivates RhoA following furrow ingression, is required for actin filament disintegration during late cytokinesis. The GTPase Rab35 and its effector, the phosphatidylinositol-4,5-bisphosphate 5-phosphatase OCRL, also regulate actin disassembly. More research is needed to understand the mechanism by which the plasma membrane eventually breaks.
Timing cytokinesis
Cytokinesis must be timed to occur only when sister chromatids split during the anaphase phase of normal proliferative cell divisions. Many components of the cytokinesis machinery are strictly controlled to ensure that they can only perform a certain role at a specific stage of the cell cycle cytokinesis. Only when APC attaches to CDC20 can cytokinesis occur. This enables chromosomal separation and myosin to function together.
As the cell cycle returns to interphase after cytokinesis, non-kinetochore microtubules restructure and dissolve into a new cytoskeleton.
Facts about Cytokinesis
- Cytokinesis is the division of cells that occurs following mitosis or meiosis I and II.
- The cytoplasm (the liquid core of the cell that holds the organelles in place) breaks into two equal halves during cytokinesis, and the cell divides into two daughter cells. This occurs shortly after the onset of anaphase (in mitosis and in meiosis I and II).
- During telophase (in mitosis and meiosis I and II), cell division continues until the cell has fully divided. After cytokinesis, mitosis and meiosis II resume. In each of the two cells, a new and complete nucleus has developed.
Plant Cell
Because plant cells have a cell wall, cytokinesis differs greatly from that of animal cells. Plant cells build a cell plate in the center of the cell rather than a contractile ring. The stages of cell plate formation are as follows: (1) formation of the phragmoplast, an array of microtubules that guides and supports cell plate formation; (2) trafficking of vesicles to the division plane and their fusion to generate a tubular-vesicular network; (3) continued fusion of membrane tubules and their transformation into membrane sheets upon deposition of callose, followed by deposition of cellulose and other cell wall components; and (4) recycling of the cell plate’s surplus membrane and other material; and (5) union with the mother cell wall
The phragmoplast is made up of mitotic spindle remains and acts as a route for vesicle trafficking to the phragmoplast midzone. These vesicles contain the lipids, proteins, and carbohydrates required to build a new cell border. The Golgi apparatus has been identified as the source of these vesicles by electron tomography investigations, although other researchers have revealed that they also include endocytosed material.
Forces
Animal cells
Type II Myosin ATPase drives cytokinetic furrow ingression. Because myosins are recruited to the medial area, the contractile forces acting on the cortex have the appearance of a ‘purse string’ constriction drawing inwards. This causes an inward constriction. Because of its tight interaction with the cortex via crosslinker proteins, the plasma membrane. The overall surface area should be raised by feeding the plasma membrane via exocytosis to the constriction of the cleavage furrow.
Proteins Involved in Cytokinesis
The contractile ring is a huge cortical complex that includes actin, myosin, septins, and other proteins that arrange these cytoskeletal components. It is often considered that membrane-associated proteins attach the contractile ring to the cell surface. The contractile ring is thought to be analogous to other cortical-actin structures in the cell, such as focal adhesions and the stress fibers that accompany them.
Actinomycin D
Actin’s involvement in cytokinesis has long been acknowledged and is supported by various findings. (1) Actin is localized in a ring at the site of division in practically all cell types. (2) Cytokinesis is inhibited by anti-actin medicines like cytochalasin. (3) Budding yeast actin mutants create enormous multinucleate cells with cytokinesis and polarity abnormalities. (4) In numerous species, mutations in genes encoding a variety of actin-associated proteins affect cytokinesis.
Myosin
Cytoplasmic myosin II, the cytoplasmic (non-muscle) variant of classical long-tailed myosin, has long been thought to be the cytokinesis molecular motor. Myosin activity is an apparent candidate for a primary control point during cytokinesis due to the strict regulation of motile activity and multimer formation caused by phosphorylation of critical amino acids. A cytoplasmic myosin II molecule is a hexamer comprised of two of three polypeptide pairs: the 200 kDa heavy chain (MHC), the 18 kDa essential light chain (ELC), and the 20 kDa regulatory light chain (RLC).
Septins
The genomes of most eukaryotic animals contain several genes encoding various members of a family of related proteins known as septins, which play a general function in cell surface events involving interactions between the plasma membrane and the cortical cytoskeleton (including cytokinesis). Septin proteins have P-loop consensus sequences suggestive of nucleotide-binding activity, as well as certain family-specific motifs, and most have a C-terminal coiled-coil domain.
Additional proteins
A variety of additional cytoskeletal proteins are found near the cleavage furrow, but their role in cytokinesis is unclear. Actin filament cross-linking or bundling proteins like a-actinin, filamin, and the recently discovered anillin; actin-membrane linkers like talin and ERM proteins; those that regulate F-actin assembly or organization in other ways like acidic calponin, which inhibits the ATPase activity of phosphorylated myosin; and transmembrane glycoproteins like CD43 It is crucial to note that there is no ultrastructural evidence that majority of these proteins are contractile ring components.
Answer the quiz below to check what you have learned so far about cytokinesis.
References
- Allen, C. E. (n.d.). On the Origin and Nature of the Middle Lamella. Botanical Gazette. https://doi.org/10.1086/328131
- CYTOKINESIS | Meaning & Definition for UK English | Lexico.com. (n.d.). Lexico Dictionaries | English. Retrieved May 29, 2022, from https://www.lexico.com/definition/cytokinesis
- Cytokinesis Proteins Research Tools—Creative BioMart—Creative BioMart. (n.d.). Retrieved May 29, 2022, from https://www.creativebiomart.net/researcharea-cytokinesis-proteins_923.htm
- Fededa, J. P., & Gerlich, D. W. (2012). Molecular control of animal cell cytokinesis. Nature Cell Biology, 14(5), 440–447. https://doi.org/10.1038/ncb2482
- Meiosis | Definition, Process, & Diagram | Britannica. (n.d.). Retrieved May 29, 2022, from https://www.britannica.com/science/cytokinesis
- Mishima, M., Pavicic, V., Grüneberg, U., Nigg, E. A., & Glotzer, M. (2004). Cell cycle regulation of central spindle assembly. Nature, 430(7002), 908–913. https://doi.org/10.1038/nature02767
- Mitosis / cell division | Learn Science at Scitable. (n.d.). Retrieved May 29, 2022, from http://www.nature.com/scitable/definition/mitosis-cell-division-47
- Mokobi, F. (2022, March 30). Cytokinesis- Definition and Process (in animal and plant cells). Microbe Notes. https://microbenotes.com/cytokinesis/
- Otegui, M. S., Mastronarde, D. N., Kang, B. H., Bednarek, S. Y., & Staehelin, L. A. (2001). Three-dimensional analysis of syncytial-type cell plates during endosperm cellularization visualized by high resolution electron tomography. The Plant Cell, 13(9), 2033–2051. https://doi.org/10.1105/tpc.010150
- Otegui, M., & Staehelin, L. A. (2000). Cytokinesis in flowering plants: More than one way to divide a cell. Current Opinion in Plant Biology, 3(6), 493–502. https://doi.org/10.1016/s1369-5266(00)00119-9
- Polar bodies—More a lack of understanding than a lack of respect—PubMed. (n.d.). Retrieved May 29, 2022, from https://pubmed.ncbi.nlm.nih.gov/21268179/
Polarized cytokinesis in vacuolate cells of Arabidopsis—PubMed. (n.d.). Retrieved May 29, 2022, from https://pubmed.ncbi.nlm.nih.gov/11880633/ - ©BiologyOnline.com. Content provided and moderated by Biology Online Editors.