Dehydration reaction
n., plural: dehydration reactions
[dihaɪˈdɹeɪʃən ɹiˈækʃən]
Definition: A reaction wherein water molecule forms as a byproduct, thus, a reaction that loses water
Table of Contents
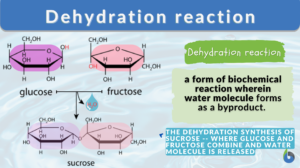
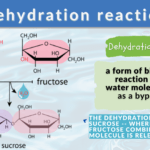
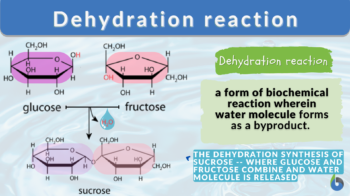
What is dehydration synthesis? A dehydration reaction is a form of biochemical reaction wherein a water molecule is lost or removed from the reacting molecules. Dehydration synthesis, in particular, is the building up of compounds or molecules while losing water molecules.
In particular, it is a type of condensation reaction in which the monomers join together into polymers while losing water molecules. In contrast, the addition of a water molecule into a compound or a substance is called a hydration reaction.
Hydrolysis, in particular, is the process of combining a water molecule with a compound, e.g. a disaccharide, which when hydrolyzed converts into two monosaccharides. Let’s define dehydration synthesis to further understand it. Read more information below.
Dehydration Synthesis Definition
Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water molecule or water molecules.
What is the result of a dehydration reaction?
Dehydration synthesis leads to the formation of polymers from small monomers where monomers are combined together by the removal of small molecules such as water. Dehydration reaction results in the formation of a covalent bond. Therefore, it is a type of condensation reaction. Thus, water is a common byproduct of condensation reactions in biological systems.
A common example of dehydration reactions is the formation of ethers from the condensation of alcohols. This reaction takes place in an acidic medium since it is catalyzed by the presence of an acid. This reaction is reversible. Therefore, dehydrating chemicals are used to collect water from the system so that the reaction can proceed in one direction only.
Watch this vid about dehydration reaction and how it is biologically important:
Biology definition:
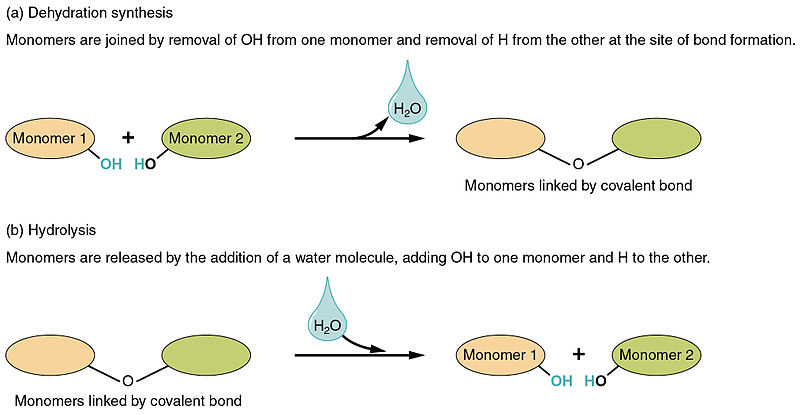
A dehydration reaction is a chemical reaction wherein a water molecule is lost, such as that during the synthesis of an organic compound. In biology and organic chemistry, synthesis is the process of creating an organic compound, especially through the aid of enzymes. One way of creating such compounds is through the loss of water molecules, a process loosely called dehydration. In biology, this process is demonstrated when a monomer loses -OH and another monomer loses H, the two unstable monomers join together, and the -OH and H combine forming water (H2O).
i.e., A-OH + B-H → AB + HOH
Etymology: Latin dē-, meaning “remove” + Ancient Greek ὕδωρ (húdōr), meaning “water”) and Latin reāctiō, reagō, i.e., re-, meaning “again” + agō, meaning “to act”
Compare: hydrolysis
See also: dehydration, synthesis
Types of Dehydration Synthesis
There are the types of dehydration synthesis reactions depending on what happens in a dehydration reaction…
- Biochemical reactions ”based on the nature of the reactants”: it is a dehydration synthesis example in which the reaction of peptides forms proteins. As amino acid is the building block of proteins. Amino acids have two functional groups — amino group (NH2) and carboxyl groups COOH. Proteins are formed as one amino group (NH2) of an amino acid reacts with the hydroxyl group (COOH) of another amino acid and forms amide linkage which will result in releasing a water molecule as a byproduct.
- Condensation reactions: the condensation reaction definition is the formation of a polymer from small monomers such as the reaction of polysaccharides formation from monosaccharides monomers. Each monomer is a polyhydroxyl carbonyl molecule that attaches to the next one by the formation of a glycosidic bond between the hydroxyl group of one monomer and the carbonyl group of another monomer and water is released from this reaction during the formation of the glycosidic bond. Compounds dehydration reaction example is using glucose to synthesize maltose and glycogen.
- Modification reaction: it is a type of reaction where the dehydration process plays an important role in the modification of compounds such as phosphorylation and glycosidation of compounds. This process is important for the modification of proteins, nucleosides, and carbohydrates by glycosylation or phosphorylation. Modification reactions help in the regulation of protein kinase cascade in the human body to perform different functions.
Dehydration synthesis reactions may be classified according to the type of the used catalyst. Because most dehydration reactions are reversible, a catalyst is used to drive the reaction in one direction only. Examples of conditions changed as a result of using catalysts are salt concentration, pH, temperature, and water availability in the system.
What is the Substitution Reaction?
Substitution reaction is the reaction at which one group of one reactant is replaced by another group of another reactant to form two new compounds. There are two main types of substitution reaction as the following;
- Nucleophilic reaction: a nucleophilic atom or molecule carries a negative charge. This molecule can interact strongly with a positively-charged molecule. This is called a nucleophilic reaction. One of the most important examples to explain this reaction is the reaction of any alkyl halide with a strong base where the alkyl halide reacts with the nucleophile forming a new bond at the alpha-carbon. An alcohol is formed and the halogen then is released and is called a leaving group.
- Electrophilic reaction: The electrophile is the atom or molecule which has a positive charge or can donate the electron and interact strongly with a negatively-charged molecule or atom. Examples of electrophilic substitution reactions are:
- Electrophilic aromatic substitution: at which the electrophile interacts with an aromatic ring by displacement of the hydrogen atom from the ring.
- Electrophilic aliphatic substitution: as the electrophile will displace one functional group from the aliphatic chain like beta-hydroxy ketone dehydration
Examples of Dehydration Synthesis
Dehydration synthesis reactions are numerous, they are included in many industries as well as biological systems.
Examples of dehydration synthesis include:
Polyester synthesis
Polyester is commonly used in making yarns, ropes, bottles, and insulting tapes. Polyethylene is one of the most common polyesters. Polyethylene terephthalate (PET) is a polymer used in the manufacture of bottles that can be recycled. PET is one of the most common examples of using dehydration synthesis in the industry as it is formed from united monomers of ethylene glycol and terephthalic acid.
Alcohol dehydration
Alcohols may undergo a dehydration synthesis process in the presence of concentrated acids like concentrated sulfuric acid, concentrated phosphoric acid, and hot aluminum oxide which act as dehydrating agents. One example of an alcohol dehydration reaction is known as acid-catalyzed dehydration.
Examples of dehydration of alcohols to alkenes:
- dehydration of ethanol: (CH3CH2OH) can be dehydrated to form ethene (C2H4) at 170 ℃.
- 2-butanol (C4H9OH) can be dehydrated to form 2-butene (C4H8).
- dehydration of Cyclohexanol: (C6H5OH) can be dehydrated to form cyclohexane (C6H4).
Carbohydrates dehydration
Carbohydrates dehydration which is referred to as sugar dehydration reaction is the most common example of dehydration synthesis reaction as carbohydrates are mainly formed by the combination of monomers to form disaccharides or polysaccharides.
Examples of carbohydrates dehydration synthesis are:
- Two sugars of glucose (C6H12O66) are combined together by dehydration synthesis to form maltose sugar (C12H22O11)
- Two different sugars of glucose and lactose sugar can interact with each other by dehydration synthesis to form lactose.
- Glucose and fructose interact by dehydration synthesis to form sucrose.
Amino acid
Amino acids are examples of polypeptide dehydration synthesis. Amino acids are combined with each other through peptide bonds which form polypeptide making chains of proteins. For example, in polypeptide protein dehydration synthesis, glycine can undergo dehydration and interact with itself to form a chain of the dipeptide of glycylglycine and so on.
Aldol dehydration or condensation
Aldol dehydration or condensation is dehydration in organic chemistry in which enol can interact with the carbonyl group which leads to beta-hydroxy ketone synthesis.
Cholesterol
It can undergo dehydration to form alkenes. This process is called the cholesterol dehydration reaction.
Formation of glycosidic bonds
Glycoside or carbohydrate is a simple ring of sugar molecule which is formed of 5 or 6 carbon atoms. The interaction between two glycoside rings results in the formation of a covalent glycosidic bond.
For example, sucrose is formed by the interaction of glucose and fructose, water is released during the dehydration reaction and a glycosidic bond is formed between them. Consequently, glucose polymers such as glycogen, cellulose, and starch are formed in the same manner by condensation of small sugar molecules and the formation of glycosidic bonds between them.
Glycosidic bonds are formed chemically by a condensation reaction where one water molecule is released during the reaction. A condensation reaction occurs when the hydroxyl group (OH) attacks the anomeric carbon of a sugar.
Anomeric carbon is the carbon in a sugar ring that is found between two oxygen atoms and attached by a single bond to each oxygen atom- releasing water molecule and forming a glycosidic bond between the oxygen atom in the ring of sugar and the other oxygen which is found in the hydroxyl group. This linkage chemically forms an ether linkage as the oxygen attaches to two atoms of carbon of each ring of sugars.
Are all glycosidic bonds the same? There are three different types of glycosidic bonds. They are the N-glycosidic bond, S-glycosidic bond, and O-glycosidic bond. DNA dehydration synthesis or RNA dehydration synthesis are biochemical examples of glycosidic bonds, as sugar units are connected to nucleobases by N-glycosidic bonds. Moreover, glucuronic acid attaches to different substances by a glycosidic bond to increase their water solubility to facilitate excretion and metabolism inside animals’ bodies.
Triglyceride formation
How are triglycerides formed chemically?
Triglycerides are chemically formed by connecting three carboxylic groups of a glycerol molecule with three carboxylic groups of a fatty acid-forming ester bond with the release of three water molecules. The length of triglycerides may vary depending on the length of the fatty acid chain between 16 to 20 carbon moieties.
Therefore, This reaction is chemically considered a condensation reaction. Triglyceride can be metabolized by the hydrolysis of the ester bond which is connected between glycerol and fatty acids by pancreatic lipase enzymes and this hydrolysis is done to provide energy for the body.
Triglyceride is one of the most abundant fats in the body. Triglyceride can be found and detected as a ratio in the bloodstream. The reason for their existence in the bloodstream is to supply cells with energy and may be stored in the body to give energy when needed.
Animals and humans store triglycerides as solid fats whereas plants store triglycerides as liquid oils. Triglycerides stores cause obesity in humans, therefore, the higher the triglycerides level in the bloodstream, the higher the risk of obesity. Elevated levels of triglycerides in the blood are medically known as hypertriglyceridemia and this condition directly increases the risk of cardiovascular diseases and metabolic syndrome.
How can you detect if the triglyceride is harmful or harmless?
Triglyceride ratio can be detected by blood tests in mmol/L as follows:
- Normal level: less than 1.7 mmol/L. This normal level is useful in energy usage of the body
- Borderline level: if the ratio is between 1.7 to 2 Mmol/L. when this level is reached, dietary modifications should be done to avoid further elevation
- High level: if the ratio exceeds 2 to 6 Mmol/L
- Very high level: if the ratio is more than 6 Mmol/L
How can triglyceride be higher than the normal ratio?
- Overeating habits
- Alcohol consumption
- Uncontrolled diabetes
- Hormonal abnormalities
- Medication usage
- Kidney disease
- Liver disease
- Genetic disorders
What Happens in Dehydration Synthesis Reaction?
A dehydration synthesis reaction is a process in which a condensation reaction takes place. Dehydration synthesis reaction leads to the formation of big molecules from two small molecules with the elimination of water molecules.
A dehydration reaction is a process in which reactants lose one oxygen atom and two hydrogen atoms in the form of water molecules or molecules. Consequently, water is a byproduct of dehydration reaction, other products of dehydration reaction are usually polymers which form due to the connection of two reactants and the formation of a double bond or a ring structure in organic molecules.
On the other hand, molecules of inorganic chemistry that are involved in dehydration reactions are usually hydrates which are not covalently bonded to water molecules but complexed to them.
Dehydration is usually reversible, they proceed in dehydration and hydrolysis pathways as long as water is available in the system. Therefore, dehydrating agents are chemicals commonly used to shift the reaction to the dehydration pathway.
There are many examples of dehydration synthesis processes such as the formation of di or polysaccharides from monosaccharides. Another example is the formation of ether from dehydration of alcohols.
Dehydration Synthesis and Hydrolysis
Dehydration synthesis is a chemical reaction in which a condensation reaction takes place with the elimination of one or more water molecules. On the other hand, hydrolysis is a hydration reaction that reverses the dehydration reaction. Hydrolysis is the process at which there is a cleavage of bonds of large molecules to form small molecules with the introduction of water.
To sum up, the main differences between dehydration synthesis and hydrolysis chemical reactions are shown in the table below:
Dehydration synthesis vs. Hydrolysis | |
|---|---|
| Dehydration synthesis | Hydrolysis |
| Definition: the process of eliminating water and forming large molecules. It is the opposite of hydrolysis | Definition: the process of consuming water and breaking the bond of large molecules to form small molecules. |
| Mechanism: combination or condensation reaction. | Mechanism: decomposition reaction |
| Role of water: It is a byproduct | Role of water: It is a reactant |
Data Source: Nadine Omar of Biology Online
Frequently Asked Questions on Dehydration Synthesis
- What is the difference between dehydration and hydrolysis reactions?
Hydrolysis is a word that refers to water cleavage. So, in dehydration synthesis reactions, compounds lose water, while in hydrolysis, water is consumed in the reaction. - Which molecules can undergo dehydration reaction?
Organic molecules contain double or triple bonds and inorganic hydrant molecules. - What is dehydration synthesis reaction?
Dehydration synthesis is a type of condensation reaction that results in the combination of two or more molecules by the removal of water. - What is the importance of dehydration reaction?
A dehydration reaction is used in the biosynthesis of molecules such as triglycerides, polysaccharides, and proteins. Dehydration synthesis is important in industries such as insulating papers and recyclable bottles. - What is the role of dehydration reaction in organic chemistry?
A dehydration reaction is used in the following interactions:- Esterification
- Nitrite formation
- Etherification
- Formation of alkenes
Answer the quiz below to check what you have learned so far about dehydration synthesis.
References
- Brent Cornell. BioNinja. (n.d.). Retrieved June 28, 2022, from https://ib.bioninja.com.au/standard-level/topic-2-molecular-biology/23-carbohydrates-and-lipids/triglycerides.html
Dehydration synthesis: Definition, examples, and equations. Chemistry Learner. (2020, November 5). Retrieved June 28, 2022, from https://www.chemistrylearner.com/chemical-reactions/dehydration-synthesis - Glycosidic Bond. Glycosidic_bond. (n.d.). Retrieved June 28, 2022, from https://www.bionity.com/en/encyclopedia/Glycosidic_bond.html
- Libretexts. (2020, August 11). Triglycerides. Chemistry LibreTexts. Retrieved June 28, 2022, from https://chem.libretexts.org/Bookshelves/Biological_Chemistry/Supplemental_Modules_(Biological_Chemistry)/Lipids/Glycerides/Triglycerides
- Madhusha. (2017, August 19). Difference between dehydration synthesis and hydrolysis: Definition, mechanism, examples. Pediaa.Com. Retrieved June 28, 2022, from https://pediaa.com/difference-between-dehydration-synthesis-and-hydrolysis/
- Sison, J. (2015, April 15). Difference between hydrolysis and dehydration synthesis. Difference Between Analogous Terms and Objects. Retrieved June 28, 2022, from http://www.differencebetween.net/science/health/difference-between-hydrolysis-and-dehydration-synthesis/
- Smith, Y. (2021, February 17). What are triglycerides? News. Retrieved June 28, 2022, from https://www.news-medical.net/health/What-are-Triglycerides.aspx
- Take online courses. earn college credit. Research Schools, Degrees & Careers. Study.com | Take Online Courses. Earn College Credit. Research Schools, Degrees & Careers. (n.d.). Retrieved June 28, 2022, from https://study.com/academy/lesson/dehydration-synthesis-definition-reaction-examples.html
- Take online courses. earn college credit. Research Schools, Degrees & Careers. Study.com | Take Online Courses. Earn College Credit. Research Schools, Degrees & Careers. (n.d.). Retrieved June 28, 2022, from https://study.com/academy/lesson/glycosidic-bond-definition-formation-quiz.html
- Vedantu. (2022, April 27). Substitution reaction. VEDANTU. Retrieved June 28, 2022, from https://www.vedantu.com
- Organic Chemistry. (n.d.). Retrieved June 28, 2022, from https://instruct.uwo.ca/chemistry/373f/Nifty%20Stuff/Aldol%20Reaction.htm
- Take online courses. Earn college credit. Research Schools, Degrees & Careers. Study.com | Take Online Courses. Earn College Credit. Research Schools, Degrees & Careers. (n.d.). Retrieved June 28, 2022, from https://study.com/academy/answer/draw-the-structure-of-cholesterol-after-dehydration.html©BiologyOnline.com. Content provided and moderated by Biology Online Editors.