n., diˈneɪ tʃəræʃən
A process where a molecule denatures as a result of exposure to a denaturing agent
Table of Contents
Denaturation Definition
In biochemistry, denaturation is defined as a process in which a molecular structure deviates from its original state when exposed to a denaturing agent. In biology, examples of biomolecules that denature are proteins and nucleic acids (e.g. DNA). A denatured protein, for instance, means a protein whose three-dimensional (3D) structure is disrupted due to exposure to certain chemical or physical factors (called denaturants). Denaturants may be in the form of heat, radiation, acid, solvents, etc. When a protein is exposed to a denaturant, its structure is altered resulting in the loss of its innate biological activity and function. Apart from protein, nucleic acid, such as DNA, could also be denatured. Exposing DNA to heat, for instance, could cause its 3D structure to change. From the original double-stranded state, it may turn into a single-stranded molecule due to the dissociation of the two strands by heating.
In the food industry, denaturation is defined as the process of making food or drink (e.g. alcohol) unfit for human consumption by the deliberate adulteration of food or drink, e.g. by the addition of a noxious substance.
Etymology
The term denaturation is a combination of “denature” and suffix –”ion”. The word denature came from the French “dénaturer”, which, in turn, came from Latin “dis”-, meaning “apart”, and “nātūra”, for “nature”.
Types of Denaturation
Denaturation types may be based on the cause: (1) biologically-induced or (2) non-biologically-induced.
Biologically-induced denaturation
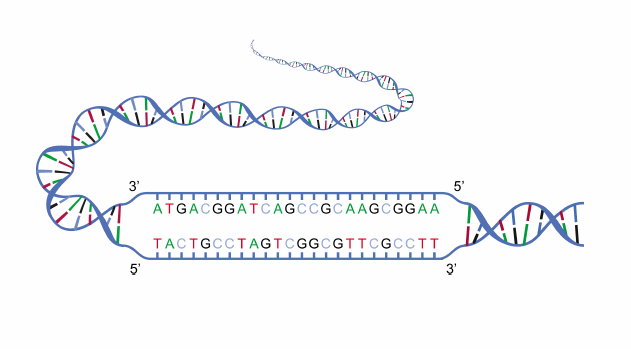
This is a form of denaturation that occurs in biological systems. This includes the biologically important DNA processes, such as DNA replication, transcription, DNA repair. In these processes, the double-stranded DNA unwinds and the two strands become partially separated forming the so-called “bubble”. In transcription, for instance, part of the DNA unwinds and form a transcription bubble from where a site in the bubble serves as the docking site for RNA polymerase. (Ref.1)
Non-biologically-induced denaturation
Non-biologically-induced denaturation entails a process that is not biological in nature but is caused by other external means, which can be chemical or physical. Chemicals, such as heavy metals and metalloids can disrupt biomolecular structures. They cause protein denaturation in different ways. For example, proteins are denatured when these chemicals react through their functional side groups or when they oxidize the amino acid side chains. They may also tend to dislocate metal ions in metalloproteins. (Ref.2) Apart from directly disrupting protein structure, chemicals (acids or bases) may also induce denaturation by changes in pH. Acids that cause the physiological pH to drop to 5 or lower can lead to the so-called acid-induced protein unfolding; bases that cause the pH to rise to 10 or higher may possibly lead to base-induced protein unfolding. (Ref.3) As for physical factors, environmental stress that can cause protein and nucleic acid denaturation include extreme temperature, radiation, salinity, pressure, and electronegative molecules in the atmosphere (e.g. nitrogen and oxygen); they can break the hydrogen bonds in a biomolecule.
Effects of Denaturation in Biomolecules
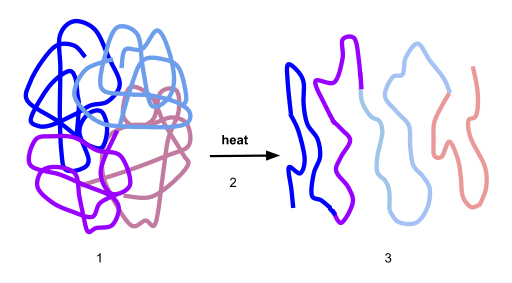
The presence of denatured proteins or denatured nucleic acids implies a potential disruption in cell activity. If the cell fails to correct the disturbance it is, then, at risk of early cell death. The proteins can regain their natural active state if the denaturing agent is removed. However, there are instances in which the process is irreversible. Proteins have to be properly folded in order to become functional. Nucleic acids, in turn, have to maintain structural stability to minimize the potential risk of mutation. Otherwise, the body could be at risk of developing a disease. Protein denaturation and aggregation accounts for blindness, Alzheimer’s disease, and many other neurodegenerative diseases. (Ref.4)
Denaturation that entails a change in chemical structure is not applicable to explaining alcohol denaturation in the food industry. It is a different aspect. Denaturation in this regard does not involve a change in structure but merely the addition of a noxious substance to a food or drink. The chemical structure of denatured alcohol, for example, is not altered; rather, it just means it has additives to make it unpalatable to humans.
Functions of Denaturation
At the cellular level, denaturation is a crucial step, particularly in many DNA processes (as described above). It partially “opens” the DNA and allows replication or transcription to proceed. Otherwise, DNA strands may not be copied in preparation for mitosis or in creating an mRNA transcript for protein translation. At the level of the biological system, denaturation is used by the body to kill pathogens. It does so through pH regulation and biochemical secretions. Denaturation is also important during food digestion. Proteins in food are denatured by the action of the released digestive enzymes.
In the medical field, the same principle is applied when using a denaturation mechanism in killing bacteria, viruses, and other cells causing diseases. In laboratory and research, denaturation is a favored process in a polymerase chain reaction, a technique used to produce several in vitro copies of DNA quickly.
Related Terms
See Also
References
- Transcription (biology) | Biology Articles, Tutorials & Dictionary Online. (2019). Retrieved from Biology Articles, Tutorials & Dictionary Online website: https://www.biologyonline.com/dictionary/transcription-biology/
- Tamás, M. J., Sharma, S. K., Ibstedt, S., Jacobson, T., & Christen, P. (2014-03-04). “Heavy Metals and Metalloids As a Cause for Protein Misfolding and Aggregation”. Biomolecules. 4 (1): 252–267.
- Konermann, L. (2012-05-15). Protein Unfolding and Denaturants. eLS. Chichester, UK: John Wiley & Sons, Ltd.
- Meredith, S. C. (2005). Protein denaturation and aggregation: Cellular responses to denatured and aggregated proteins. Annals of the New York Academy of Sciences, 1066, 181–221. https://doi.org/10.1196/annals.1363.030
© Biology Online. Content provided and moderated by Biology Online Editors.