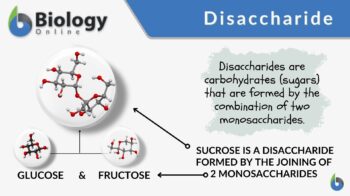
Disaccharide
n., plural: disaccharides
[daɪˈsækəɹaɪd]
Definition: a sugar consisting of two monosaccharides
Table of Contents
Carbohydrates are organic compounds comprised of carbon, hydrogen, and oxygen, usually in the ratio of 1:2:1. They are one of the major classes of biomolecules. They are an important source of energy. They also serve as structural components. As a nutrient, they can be classified into two major groups: simple carbohydrates and complex carbohydrates. Simple carbohydrates — sometimes referred to simply as sugar — are those that are readily digested and serve as a rapid source of energy. Complex carbohydrates (such as cellulose, starch, and glycogen) are those that need more time to be digested and metabolized. They often are high in fiber and, unlike simple carbohydrates, they are less likely to cause spikes in blood sugar.
Disaccharide Definition
The term disaccharide etymologically means two saccharides. A saccharide refers to the unit structure of carbohydrates. Thus, a disaccharide is a carbohydrate comprised of two saccharides (or two monosaccharide units). The term sugar can refer to both monosaccharides and disaccharides.
Monosaccharides are also called simple sugars since they are the most fundamental type of sugar. The term table sugar or granulated sugar actually refers to sucrose, which is a disaccharide made of two monosaccharides: glucose and fructose.
Biology definition:
A disaccharide is a carbohydrate made up of two monosaccharides by a glycosidic bond. Thus, a disaccharide would be able to yield two monosaccharide units on complete hydrolysis. An example of a disaccharide is sucrose, which is made up of glucose and fructose. Etymology: Ancient Greek δίς (dís, meaning “twice”) + saccharide. Synonyms: double sugar; biose. Compare: monosaccharide, polysaccharide
Characteristics of Disaccharides
Similar to other carbohydrates, disaccharides are comprised of hydrogen, carbon, and oxygen, and the ratio of hydrogen atoms to oxygen atoms is often 2:1, which explains why they are referred to as hydrates of carbon. The general chemical formula of disaccharides is C12H22O11. Because of the presence of carbon and C-C and C-H covalent bonds, disaccharides are also organic compounds, just like the other carbohydrates.
A disaccharide is a carbohydrate or a sugar comprised of two monosaccharides joined together by a glycosidic bond (or glycosidic linkage). Monosaccharides are the most fundamental type of carbohydrate. Glycosidic bonds are covalent bonds that may form between the hydroxyl groups of two monosaccharides. Thus, even if they have the same chemical formula, there are different kinds of disaccharides that differ in bond formations, as well as monosaccharide constituents, and therefore, different properties.
Disaccharides differ from other forms of carbohydrates, oligosaccharides, and polysaccharides, in the number of monosaccharide units that make them up. Disaccharides are made up of only two whereas oligosaccharides are made up of three to ten monosaccharides. Polysaccharides, as the name implies, contain several monosaccharide units.
Synthesis of Disaccharides
The chemical process of joining monosaccharide units is referred to as dehydration synthesis since it results in the release of water as a byproduct. Disaccharides are formed by displacing a hydroxyl radical from one monosaccharide and a proton from the other monosaccharide and then causing the two monosaccharides to covalently link together.
The detached hydroxyl radical and proton (hydrogen ion), in turn, join and form a water molecule. Thus, one way of synthesizing a disaccharide is through the condensation of two monosaccharides.
A disaccharide may be reverted to its monomeric monosaccharide components through hydrolysis with the help of the enzyme disaccharidases (e.g. sucrase, lactase, and maltase for the degradation of sucrose, lactose, and maltose, respectively). While condensation reaction involves the elimination of water, hydrolysis utilizes a water molecule.
Classifications of Disaccharides
Disaccharides may be classified into reducing and non-reducing.
Reducing disaccharide
A reducing disaccharide is a disaccharide in which the reducing sugar has a free hemiacetal unit that may serve as a reducing aldehyde group. Examples of reducing disaccharides are maltose and cellobiose.
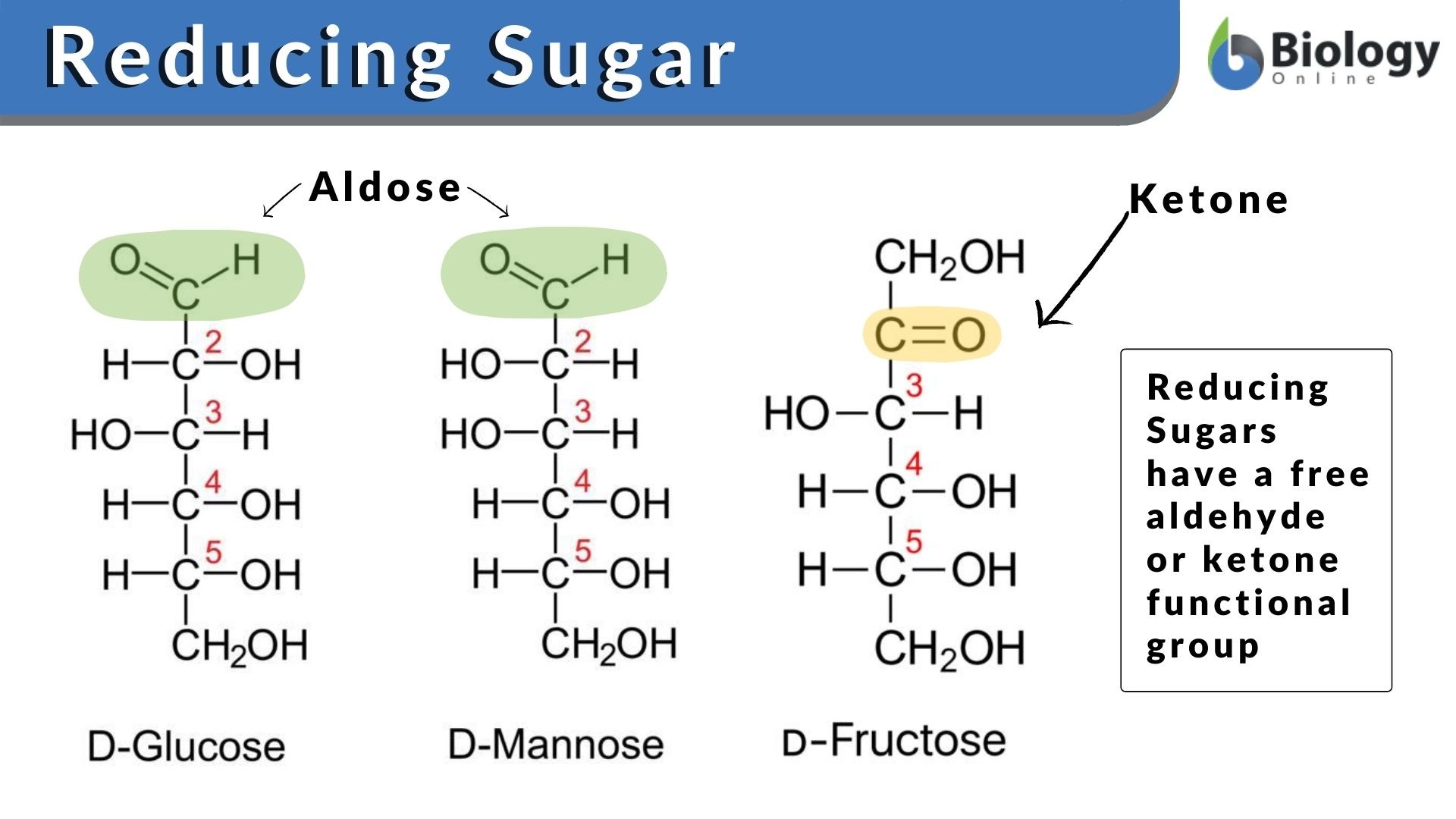
Non-reducing disaccharide
A non-reducing disaccharide, as its name implies, is a disaccharide that does not act as a reducing agent. Both monosaccharides that make up the disaccharide do not have a free hemiacetal unit since they bond through an acetal linkage between their anomeric centers. Examples are sucrose and trehalose.
Common Disaccharides
There are several forms of disaccharides but the most common ones are sucrose, lactose, and maltose. These three are made up of two monosaccharides joined by a covalent bond. The general chemical formula is C12H22O11.

Sucrose
Sucrose (common table sugar) is a disaccharide formed by the combination of glucose and fructose. These two monosaccharides combine through a condensation reaction. They are linked through a glycosidic linkage between C-1 (on the glycosyl unit) and C-2 (on the fructosyl unit). Sucrose is digested or broken down into its monosaccharide units through hydrolysis with the help of the enzyme, sucrase. The bond that joins the two monosaccharides is broken, converting sucrose to glucose and fructose. Sucrose is extracted from plants, e.g. sugar cane and sugar beet, and processed (refined) to be marketed as common table sugar. It is used as a sweetening agent in food and beverages.
Lactose
Lactose (milk sugar) is formed by the combination of glucose and galactose. It has a chemical formula of C12H22O11. Lactose is produced naturally and is present in the milk of mammals, including humans. It is collected from bovine to be used in preparing infant formulas. A cow’s milk, in particular, has about 4.7% lactose. Lactose is digested or broken down into its monosaccharide units through hydrolysis with the help of the enzyme lactase. The bond that joins the two monosaccharides is broken, converting lactose to glucose and galactose. People who are lactose intolerant cannot digest or break down lactose. This becomes food for gas-producing gut flora. This could lead to gastrointestinal disturbance and flatulence. Lactose can be converted to lactic acid. Microorganisms, such as Lactobacilli, can convert lactose to lactic acid, which is used in the food industry, e.g. in the production of dairy products like yogurt and cheese.
Maltose
Maltose (malt sugar) is a reducing disaccharide formed when two glucose monomers join together via α(1→4) glycosidic bond. Thus, it may also be considered as the structural unit of glycogen and starch. Maltose is digested or broken down into its monosaccharide units through hydrolysis with the help of the enzyme, maltase. The bond that joins the two glucose units is broken, converting maltose to two glucose units. Maltose is commercially used as a sweetener, a nutrient in infant feeding, and in bacteriological culture media. It is also used in pastries. It makes bread dough rise when carbon dioxide is produced and released during the conversion of starch into maltose by reacting the starch with enzymes.
Other disaccharides
Other examples of disaccharides are lactulose, chitobiose, kojibiose, nigerose, isomaltose, sophorose, laminaribiose, gentiobiose, turanose, maltulose, trehalose, palatinose, gentiobiulose, mannobiose, melibiose, melibiulose, rutinose, rutinulose, and xylobiose.
Biological Importance of Disaccharides
Dietary disaccharides, just as the other carbohydrates, are a source of energy. Disaccharides are consumed and digested so as to obtain monosaccharides that are important metabolites for ATP synthesis. ATPs are chemical energy biologically synthesized through aerobic and anaerobic respiration. Glucose is the most common form of monosaccharide that the cell uses to synthesize ATP via substrate-level phosphorylation (glycolysis) and/or oxidative phosphorylation (involving redox reactions and chemiosmosis).
READ: A Balanced Diet – Carbohydrates and Fat
And one of the sources of glucose is a disaccharide-containing diet. Sucrose, the common table sugar, is used commonly as a sweetener. It is used in beverages and food preparation, such as cake and cookies. When consumed, the enzyme invertase in the small intestine cleaves sucrose into glucose and fructose. Too much fructose, though, could lead to malabsorption in the small intestine. When this happens, unabsorbed fructose transported to the large intestine could be used in fermentation by the colonic flora. This could lead to gastrointestinal pain, diarrhea, flatulence, or bloating. Too much glucose could also be a health hazard.
Excessive consumption of sugar could lead to diabetes, obesity, tooth decay, and cardiovascular diseases. Lactose, a disaccharide found in breast milk, is used as a nutrient source for infants. Microorganisms, such as Lactobacilli, can convert lactose to lactic acid, which is used in the food industry, e.g. in the production of dairy products like yogurt and cheese. Maltose may be used as a sweetener although it is much less sweet than sucrose.
Vascular plants form disaccharides, especially sucrose, as a nutrient to be transported to various parts of the plant via the phloem tissues. Sugarcane, most especially, is harvested to make commercialized sugar.
Try to answer the quiz below to check what you have learned so far about disaccharides.
© Biology Online. Content provided and moderated by Biology Online Editors