Definition
noun
A molecule that receives or accepts electrons from another molecule during a redox reaction.
Supplement
An electron acceptor is an oxidizing agent and is itself reduced during the process of redox reaction.
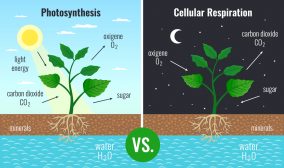
Examples of acceptors include oxygen, nitrate, iron (III), manganese (IV), sulfate, carbon dioxide, etc.
Synonym: oxidant, oxidizing agent.
See also: redox reaction, electron transport chain.
Dictionary > Electron acceptor