Endoplasmic reticulum
n., plural: endoplasmic reticula
[ˈɛndəʊˌplăz′mɪk rɪˈtɪkjʊləm]
Definition: A membrane-bound organelle in eukaryotic cells that is involved in protein synthesis, lipid synthesis, and calcium storage
Table of Contents
Endoplasmic Reticulum Definition
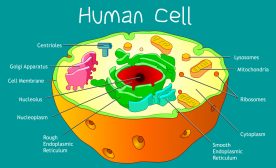
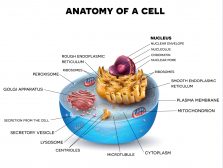
The endoplasmic reticulum is a membrane-bound organelle in cells of eukaryotic cells involved in protein synthesis, lipid synthesis, and calcium storage. There are two types of endoplasmic reticulum — the rough and the smooth type. The rough endoplasmic reticulum consists of flattened sacs and is where you will typically find ribosomes attached to its surface, thus, giving it a rough appearance under the microscope. The smooth endoplasmic reticulum, in turn, consists of tubules and vesicles and has a rather smooth appearance because ribosomes do not typically attach to its surface.
When ribosomes are seen attached to the endoplasmic reticulum, it means a protein molecule is being synthesized. Without the ribosomes, the endoplasmic reticulum is most likely involved in other cellular processes, e.g., synthesizing lipids, detoxifying or metabolizing drugs and toxins, or regulating calcium ions.
Aside from the rough and the smooth types, there are special kinds (or subtypes) of endoplasmic reticulum. Examples are the transitional endoplasmic reticulum and the sarcoplasmic reticulum. The transitional type is involved in molecular transport whereas the sarcoplasmic reticulum is primarily concerned with how to “manage” calcium ions.(Ca2+) in muscle cells.
In the next sections, we will learn more about these types and answer the common questions such as the following:
- What is the endoplasmic reticulum? What does it look like and what is it made up of? Where is it found?
- When was the endoplasmic reticulum discovered? Who discovered it?
- What is a rough endoplasmic reticulum? Is it always associated with ribosomes? Is it exclusively for protein synthesis or does it do other processes more identified with the smooth endoplasmic reticulum?
- What is a smooth endoplasmic reticulum? Is it always smooth and lacking in ribosomes? Are there times when ribosomes also attach to it? Does the smooth ER make proteins as well?
- What is a transitional endoplasmic reticulum? Why is it important?
- What is a sarcoplasmic reticulum and what is its role in muscle contraction…
- How does the endoplasmic reticulum make proteins? When and how do the ribosomes attach to the endoplasmic reticulum? Do they attach randomly? Are there signals that trigger them to attach to the endoplasmic reticulum?
- How about lipid synthesis? And how does the endoplasmic reticulum detoxify drugs and toxins?
All these will be answered as you read along!
Watch this vid about the endoplasmic reticulum ER:
The endoplasmic reticulum is a single membraned organelle that occurs as labyrinthine, interconnected flattened sacs (in the case of the rough endoplasmic reticulum) or tubules (in the case of the smooth endoplasmic reticulum) with multiple functions.
Etymology: from the Greek endon, meaning within, plasma, meaning anything formed or molded, and Latin reticulum, meaning a small net”
Abbreviation: ER
See also: rough endoplasmic reticulum, smooth endoplasmic reticulum, sarcoplasmic reticulum
Endoplasmic Reticulum Overview
Is the endoplasmic reticulum an organelle?
Yes! The endoplasmic reticulum is an organelle. Essentially, the defining feature of an organelle is that it performs a distinct function. The term “organelle” literally means the cell’s “little organ”. Another distinctive feature of a cellular structure regarded as an “organelle” is when it has a biological membrane that compartmentalizes its contents. Nevertheless, many references today consider cellular structures that are not bound by membrane “organelles”. In line with this, organelles could therefore be categorized as follows:
(1) membrane-bound organelles (included are double-membraned and single-membraned cytoplasmic structures). Examples of membrane-bound organelles are the nucleus, endoplasmic reticulum, Golgi apparatus, mitochondria, plastids, lysosomes, and vacuoles.
(2) non-membrane-bound organelles. Examples of non-membrane-bound organelles are ribosomes, spliceosomes, vaults, proteasomes, DNA polymerase III holoenzymes, RNA polymerase II holoenzymes, photosystem I, ATP synthase, nucleosome, centrioles, microtubule-organizing centers, cytoskeletons, flagella, nucleoli, stress granules, etc.
The ER is one of the three components of the GERL system, in which the Golgi apparatus and the Lysosomes are the other components.
When Was the Endoplasmic Reticulum Discovered? Who discovered it?
The endoplasmic reticulum was discovered by Albert Claude (a Belgian cytologist) and his colleagues. The invention of the electron microscope enabled scientists to explore in much higher resolutions the different cellular structures that were previously not evident using light and crude microscopes.
In 1945, Claude et al. described in their published paper (See References) a “complex anastomosing system of membranes” and what appeared to be connected to the outer membrane of the nucleus. They also talked about its role in protein and lipid synthesis.
Structure of the Endoplasmic Reticulum
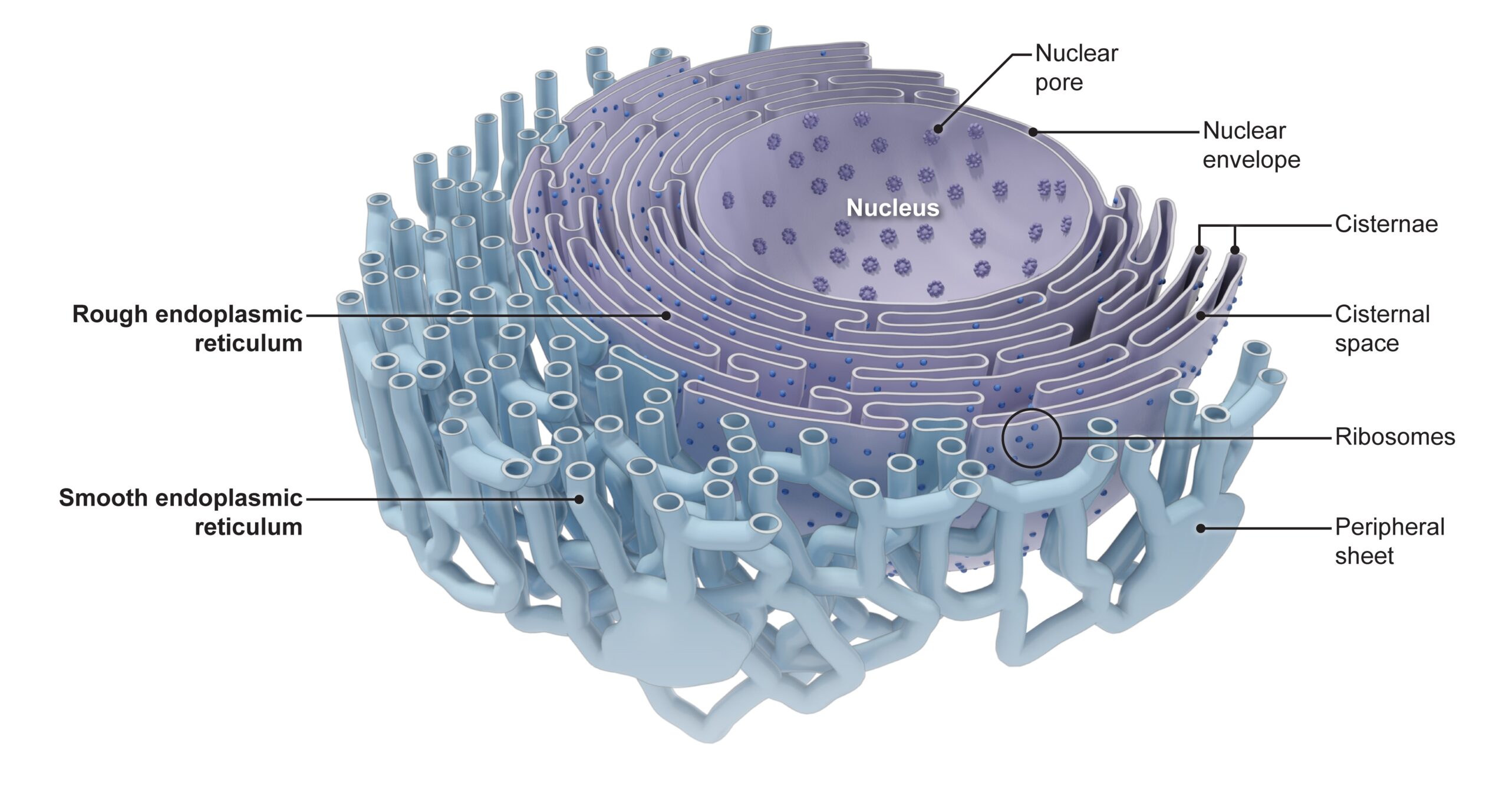
- What is the endoplasmic reticulum? What does it look like and what is it made up of?
The endoplasmic reticulum or ER is one of the most prominent organelles in the cytoplasm of a eukaryotic cell. This organelle occurs as an interconnected network of flattened sacs and tubules. The flattened sacs that are stacked on each other are called cisternae.
Now that we know the ER is a membrane-bound organelle, the next question would is this:
- Is the endoplasmic reticulum single- or double-membraned?
The endoplasmic reticulum membrane is a single membrane connected to the outer nuclear envelope (i.e., the membrane of the nucleus). Similar to other biological membranes, the membrane of the endoplasmic reticulum is a lipid bilayer, which means it has two layers of phospholipids, with various membrane proteins and carbohydrates. However, unlike the nucleus, chloroplasts, and mitochondria which have two lipid bilayer membranes (resulting in distinct outer and inner membranes), the endoplasmic reticulum has only one lipid bilayer membrane, similar to the plasma membrane.
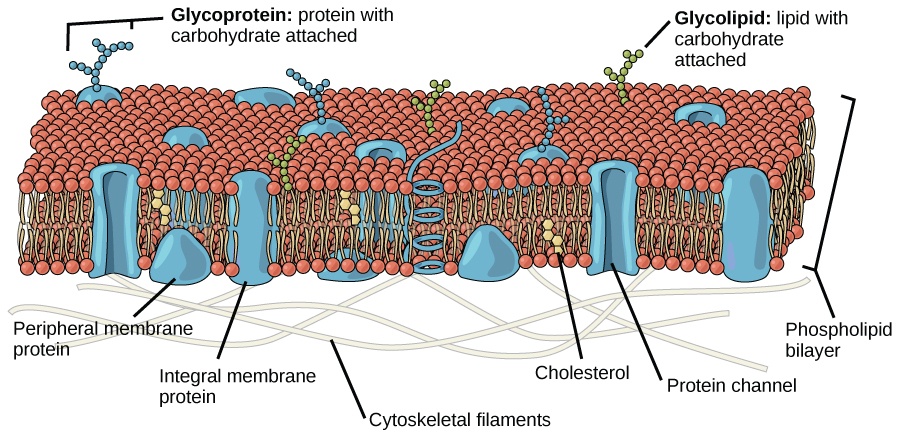
- Are ribosomes part of the endoplasmic reticulum?
No, ribosomes are cellular structures with distinct characteristics and functions. They may be seen attached to the surface of the endoplasmic reticulum where they mark the sites (locations) of protein synthesis.
- What is in the lumen of the endoplasmic reticulum?
The lumen of the endoplasmic reticulum contains various enzymes and molecular chaperones. Nevertheless, the luminal contents vary depending on what is being synthesized (protein or lipid) or carried out (e.g., whether calcium ion regulation or drug/toxin detoxification).
- When engaged in protein synthesis, the lumen will contain enzymes and chaperone proteins for protein folding, modifications, and initial stages of transport.
- For lipid syntheses, such as phospholipids (membrane lipids), phosphatidylcholine, and phosphatidylethanolamine, the lumen will likely contain greater amounts of enzymes involved in the synthesis of such lipids. To name a few, such enzymes include acyl-CoA synthetases, acyltransferases, phosphatidate phosphatase, diacylglycerol acyltransferase, and phosphatidylserine synthase.
- For metabolism and detoxification of drugs, toxins, and carcinogens, the lumen will likely contain enzymes such as cytochrome P450s (CYPs)
- When engaged in calcium ion regulation, calcium ions are stored in the lumen of the endoplasmic reticulum (specifically referred to as the sarcoplasmic reticulum)
Types of Endoplasmic Reticulum
There are two basic types (or regions) of ER: the rough endoplasmic reticulum (rough ER, or simply, rER) and the smooth endoplasmic reticulum (smooth ER, or simply, sER). The rER bears many ribosomes on its outer surface giving it a rough appearance as seen in electron micrographs; hence, its name. In contrast, the sER does not have ribosomes attached to its outer surface thus making it relatively smoother.
Rough Endoplasmic Reticulum
What is a rough endoplasmic reticulum? Is it always associated with ribosomes? Is it exclusively for protein synthesis or does it do other processes? Read on to get the answers!
The rough endoplasmic reticulum (rER) is distinguished by the following characteristics:
» It consists of flattened sacs and cisternae.
» It is studded with ribosomes.
» It is primarily involved in protein synthesis.
» It is typically closer to the nucleus than the smooth ER in most cells.
Let’s take a bit closer look at the characteristics of rER:
» Rough ER consists of flattened sacs and cisternae.
Rough ER consists of flat sheets and not tubules like the smooth ER because the sheet-like structure offers a larger surface area for ribosomes to attach, making protein synthesis more efficient.
» Rough ER is studded with ribosomes.
The presence of the ribosomes is a “telltale sign” that the endoplasmic reticulum is a rough ER. However, you have to note that ribosomes are not permanently attached to the endoplasmic reticulum. There are times when they detach from it and freely float in the cytoplasm, or attach to other organelles, such as the nucleus via the nuclear envelope. (CK-12 Foundation, 2023)
But when it’s time to make proteins, especially those that are for transport outside the cell or for use by the cell membrane, they will assemble on the surface of the rough endoplasmic reticulum.
» Rough ER is primarily involved in protein synthesis.
Together with the ribosomes, the rough ER is primarily involved in making and processing proteins. But is the rough ER exclusively for protein synthesis or does it do other than that? While rough ER is mostly about protein synthesis, there are also instances wherein it carries out processes usually associated with smooth ER, such as lipid synthesis and drug detoxification. (See Vance, 1990 and Guengerich, F. P., 2001, respectively) Nevertheless, rough ER is so much more into making proteins than doing the jobs chiefly done by the smooth ER.
» Rough ER is typically closer to the nucleus than the smooth ER in most cells.
Because the rough ER is primarily involved in protein synthesis, it is usually closer to the nucleus (where the genetic material is located) than the smooth ER is, in most cells. An example of a cell that has a smooth ER closer to the nucleus than a rough ER is a hepatocyte (liver cell) as liver cells are for the most part involved in detoxifying lipophilic drugs. (Lehninger et al., 2005)
Smooth Endoplasmic Reticulum
What is a smooth endoplasmic reticulum? Is it always smooth and without ribosomes or are there times when ribosomes also attach to it? Does the smooth ER make proteins as well? Read on to get the answers!
The smooth ER is a region in the endoplasmic reticulum that is generally “smooth” because ribosomes typically do not dock on its surface. Its membrane is continuous with the rough ER.
It may be identified by the following common features:
» It consists of tubules and vesicles.
» It typically has no ribosomes bound to it.
» It is primarily involved in lipid synthesis, detoxification, and calcium homeostasis.
» It is typically not found next to the nucleus as the rough ER is in most cells.
Rather than flattened sacs, the smooth ER consists of tubules and vesicles that branch forming a network. While the tubular shape helps to increase surface area as well, it appears to be well-suited for lipid synthesis and detoxification.
See Figure 1 to compare the smooth ER tubules and the flat sheets of the rough ER.
The smooth ER is chiefly associated with lipid synthesis, drug detoxification, and calcium storage/regulation.
Because the smooth ER is more involved in lipid synthesis, it typically contains the enzyme Glucose-6-phosphatase (which converts glucose-6-phosphate to glucose), a step in gluconeogenesis. But apart from that, recent biological chemistry and cell biology studies reveal that the smooth ER is apparently also making proteins. Certain ribosomes are bound to it under certain conditions, such as when the cell is under stress. Hetz & Saxena (2017) elucidated such rare instances in their paper:
“In response to certain cellular stresses, such as nutrient deprivation, viral infection or exposure to toxins, ribosomes can localize to the surface of the smooth ER, where they synthesize membrane-associated proteins that aid in the cellular response to stress.” (p. 482)
Other reported evidence of the rare binding of ribosomes to the smooth ER is as follows:
- Ribosomes attach to the smooth ER of liver cells (hepatocytes) during starvation-induced autophagy (Zhang et al., 2016)
- Ribosomes attach to the smooth ER of the neurons of patients with Alzheimer’s disease (Hoozemans et al. 2007)
- Ribosomes attach to the smooth ER of the proximal tubule cells of the kidney during ischemia-reperfusion injury (Liu et al., 2019)
So those are the two main types (regions) of the endoplasmic reticulum. But it doesn’t end here. There are special forms or regions of ER that have been named based on their special functions.
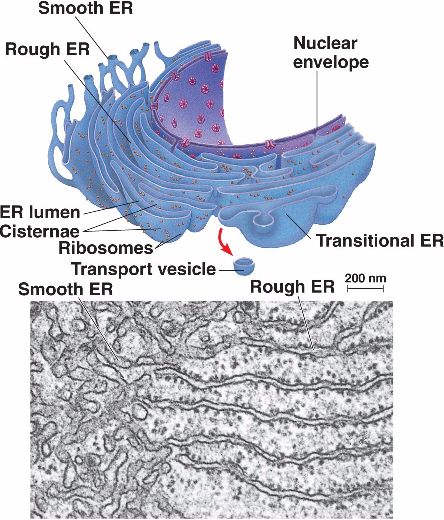
Special Regions or Other Forms of ER
Let us take a look at the other regions or forms of endoplasmic reticulum.
Transitional Endoplasmic Reticulum
What is a transitional endoplasmic reticulum? Why is it important?
The transitional ER (tER), as the name implies, is a special region in the ER that acts as a “transition” between the rough and the smooth ER — having flattened sacs or tubules but without the ribosomes. See Figure 3.
Also, transitional ER has markers, such as the vesicle coat proteins (e.g., COP I and COP II) for vesicle formation and trafficking.
The transitional ER takes the role of producing vesicles that convey the newly synthesized proteins from the rough ER (or lipids from the smooth ER) to the Golgi apparatus where they will be sorted and labeled for transport (whether inside the cell or outside the cell). Remember that the rough ER and the smooth ER are interconnected regions and therefore can allow molecules to be transported to the endomembrane system, including the Golgi apparatus. The transitional ER is therefore often seen near the Golgi apparatus, sending “cargoes” in the form of vesicles that bud off from the transitional ER.
Sarcoplasmic reticulum
What is a sarcoplasmic reticulum and what is its role in muscle contraction…
A sarcoplasmic reticulum is a special form of smooth ER in muscle cells. See Figure 4 (yellow network of tubules).
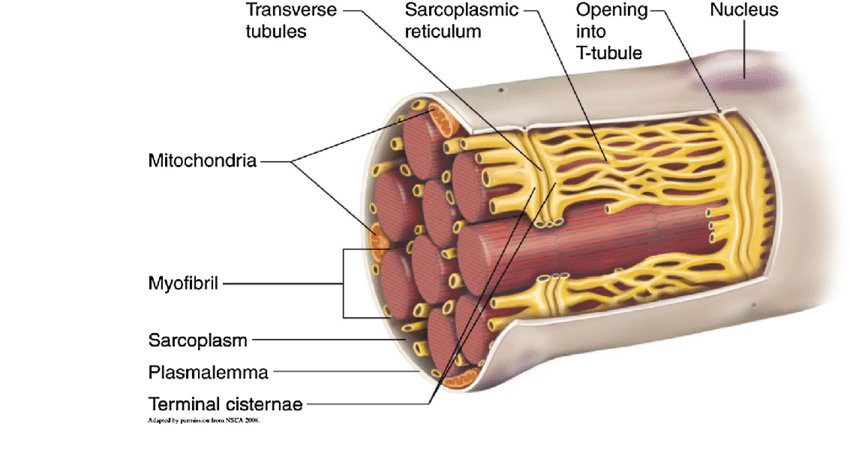
The sarcoplasmic reticulum plays a key role in the storage and release of calcium ions, which in turn, is responsible for muscle contraction (during Ca2+ release) and relaxation (during Ca2+ reabsorption).
(jump to the “ER Functions: Calcium ions transport by the sarcoplasmic reticulum…”).
Other special forms or regions
- Smooth endoplasmic reticulum of steroid hormone-producing cells. This special type of smooth ER is involved in steroid hormone synthesis, such as the synthesis of cholesterol (a precursor for steroid hormones and a component of the plasma membrane). Thus, it has a role in producing steroid hormones such as cortisol, androgens, and aldosterones.
- Peroxisomal ER. As the name implies, this is a region of the ER that is near the peroxisomes. It is responsible for the biogenesis of peroxisomes and possibly in lipid metabolism and membrane trafficking between the ER and the peroxisome.
- Nuclear envelope ER. It is another special region of the rough ER found near the nuclear envelope (membrane of the nucleus). This, in turn, is involved in the biogenesis of the nuclear envelope, including the nuclear pore complex assembly. Thus, it is most especially active during telophase, when the nuclear envelope forms around the nuclei of the newly formed cells during mitosis (and meiosis).
- Synaptic ER. It is a special name for ER of neurons that are localized near synapses. It, however, is special because of its role in neurotransmitter release and synaptic plasticity.
- Lamellar body ER. It is a special type of ER in the lungs that is responsible for the production and storage of pulmonary surfactants that reduce surface tension and help prevent the collapse of the lungs during exhalation.
Endoplasmic Reticulum Functions
Below are the primary functions of the endoplasmic reticulum:
Protein synthesis and folding
How is the endoplasmic reticulum involved in making proteins? When and how do the ribosomes attach to the endoplasmic reticulum? Do they attach randomly? Are there signals that trigger them to attach to the endoplasmic reticulum? Read on to get the answers!
Let’s have a quick recap of protein synthesis. In biological systems, the major steps of protein synthesis are amino acid synthesis, transcription, and translation. Briefly, the process of transcription is a nuclear event wherein the mRNA template, encoding the sequence of the protein in the form of a trinucleotide code, is transcribed from DNA to provide a template for translation. Translation is a cytoplasmic process and the site of translation is the ribosomes. There, the amino acids are added by tRNAs and then are linked together in a specific order as specified in the mRNA transcript. Subsequent to these events are the maturation processes, such as proteolysis, post-translational modification, and protein folding.
Now, this is where the role of the endoplasmic reticulum in protein synthesis comes in…
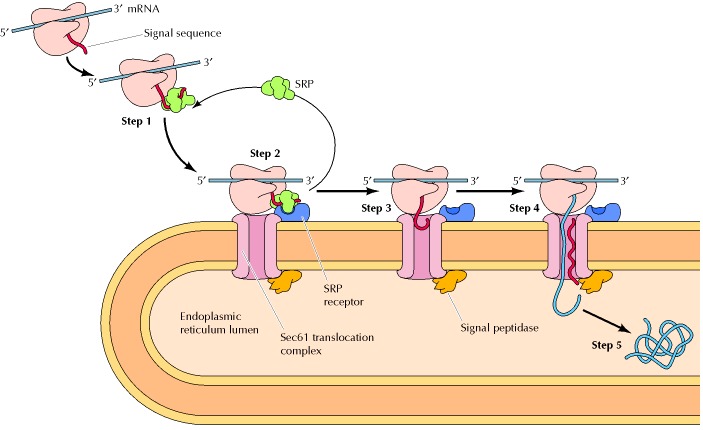
1 In the early phase of translation, a signal peptide sequence is synthesized (i.e. by protein translation at the ribosome). This signal is an indication that the protein is for further processing in the endoplasmic reticulum.
2 When this signal peptide is recognized by a signal recognition particle (SRP) in the cytosol, it escorts the ribosome+mRNA complex to the ER. The SRP binds to the special SRP receptor on the ER membrane.
3 The ribosome that translates the protein docks to the endoplasmic reticulum via the ribosome receptor, which subsequently becomes a translocon complex. The SRP detaches and goes back to the cytosol.
4 The ribosome, then, resumes the translation of the protein. The chain continues to grow as the mRNA transcript is translated through the docked ribosome. The chain eventually makes its way into the endoplasmic reticulum through the translocon that spans across the ER membranes. (This process is referred to as translocation). Ribophorin I, a transmembrane protein, assists in ensuring the proper positioning and orientation of the nascent polypeptide into the lumen of the rough ER.
5 The signal peptide is removed by a signal peptidase in the lumen of the ER.
6 The nascent protein undergoes modifications, such as folding, glycosylation, and disulfide bond formation. These processes are facilitated by the chaperone proteins (e.g. ERp29, protein disulfide isomerase, BiP/Grp78, calnexin, etc.) and enzymes residing in the lumen of the ER.
» N-linked glycosylation occurs in the rough ER. It is when the glycan chain attaches to specific asparagine residues in nascent peptides through the catalytic action of the enzyme oligosaccharyltransferases.
» The properly-folded protein will then be packed in the form of a transport vesicle to be shuttled to the Golgi apparatus to undergo further modification, maturation, and transport — whether for transport along the cytoskeleton to other cytoplasmic organelles like lysosomes and peroxisomes or for secretion out of the cell.
» For those proteins not for transport, they are retained in the ER, such as those that will become part of the ER membrane. Those that are retained will have a retention motif, e.g., KDEL (for proteins retained in the ER lumen) and KKXX (for transmembrane proteins in the ER membrane).
NOTE IT!
“Translation does not begin in the endoplasmic reticulum but it can end there!”
The translation of proteins begins on the free ribosomes. When proteins are meant for transport or for ER use, a signal peptide sequence has to be made on the ribosomes, like a “ticket” for a ride via the signal recognition particle, which takes the entire translating unit (i.e., ribosome, mRNA, and the signal peptide sequence) to the ER where the rest of the translation process will occur.
The fate of the ribosome
So what happens to the ribosome after protein synthesis?…
Remember that ribosomes are bound transiently to the endoplasmic reticulum. When it is no longer used for protein translation, the ribosome will eventually detach and return to the cytoplasm (or attach to other organelles) for the next round of protein synthesis.
If it has to bind to the ER again, will it bind to the same site or does it bind randomly?
While the binding of the ribosome to the ER is not wholly random, it is also not strictly predetermined. The exact site where it attaches will largely depend on the ‘message’ being translated and other cellular factors (such as the availability of docking sites and cellular activities at the time of translation) but not intrinsically on the ribosome itself.
Lipid synthesis and metabolism
How does the endoplasmic reticulum make lipids?
The smooth ER is the major site of lipid synthesis, particularly in areas that are in close contact between the ER and cytoplasmic organelles and plasma membrane. These areas are referred to as the membrane contact sites (MCS).
- Example: Phosphatidylserine – a phospholipid synthesis
Note that phospholipid is important in forming and maintaining the lipid bilayer of biological membranes. Here is a summary of how the smooth ER is involved in lipid synthesis, metabolism, and transport, and its interplay with other organelles:
1 In the smooth ER, the enzyme dihydroceramide synthase (a.k.a. ceramide synthase) catalyzes the addition of fatty acyl-CoA molecules to sphinganine, forming dihydroceramide. This precursor molecule is transferred from the smooth ER to the Golgi apparatus via ER-Golgi contact sites.
2 In the Golgi, the dihydroceramide is modified further, particularly by the removal of its dihydro group, converting it into a ceramide. Then, the enzyme phosphatidylserine synthase transfers the phosphatidyl head group from phosphatidylcholine to the ceramide, converting the latter into phosphatidylserine (a type of phospholipid).
3 The Golgi transports the phosphatidylserine to membrane structures, such as the mitochondria, via Golgi-derived transport carriers. These carriers bring their ‘cargo’ to the mitochondria, incorporating the phosphatidylserine into the mitochondrial membranes.
4 There are instances wherein the mitochondrial phosphatidylserine is transferred to the smooth ER via the specific protein complexes at the ER-mitochondria contact sites.
5 The smooth ER may modify phosphatidylserine, using it to synthesize another lipid, such as phosphatidylethanolamine.
6 Phosphatidylethanolamine can then be transferred to organelles, including the mitochondria. The smooth ER transports it to the mitochondria via the lipid transfer proteins at the contact sites, contributing to mitochondrial biogenesis.
Detoxification
How does smooth ER detoxify drugs and toxins?
The detoxification process is basically about making the toxic compound more water-soluble and thus, easier to eliminate from the body. Two major families of enzymes are involved: (1) cytochrome P450 enzymes and (2) UDP-glucuronosyltransferases. These enzymes are located in the membrane of the smooth ER.
- The cytochrome P450 enzymes are the ones involved in Phase I metabolism, which is aimed at making the chemicals more susceptible to modification by exposing their functional groups. This entails oxidation, reduction, and hydrolysis.
- UDP-glucuronosyltransferases are the ones involved in Phase II metabolism. Generally, they catalyze the transfer of glucuronic acid to the exposed functional groups from Phase I, converting them into glucuronide conjugates. This step (conjugation process) turns them into more water-soluble metabolites, thereby facilitating easier excretion by various elimination routes, such as urination in the kidneys and perspiration through the skin.
- Other enzymes involved in drug detoxification are glutathione S-transferases, oxidative enzymes, and transporters.
Hepatocytes (liver cells) are the primary cell types involved in drug detoxification. Other cells involved in drug metabolism but only to a certain extent include certain cells of the kidney, lungs, skin, and intestines.
Intracellular calcium store and homeostasis
The smooth ER also holds the key to calcium homeostasis within the cell. It takes up to store calcium ions in its lumen and then releases them via calcium pumps and channels to the cytosol or to the target organelles via membrane contact sites.
Intracellular calcium ion transport
Depending on the target organelle, the uptake and release of calcium ions may be driven passively (without utilizing ATP) or actively (utilizing ATP).
For instance, the smooth ER transports calcium ions to the target mitochondrion via the specific proteins and channels present in the membrane contact site, the mitochondria-associated ER membrane (MAM). In particular, the calcium ions move passively through the inositol trisphosphate receptors (IP3Rs) on the smooth ER membrane and then passively through the voltage-dependent anion channels (VDACs) on the mitochondrial outer membrane.
In essence, IP3Rs are involved in the passive transport of calcium ions whether to the cytosol or to the target organelles. However, the smooth ER makes use of an active transport mechanism, too. It uses the calcium ATPase SERCA (Sarco/Endoplasmic Reticulum Calcium ATPase) in its membrane to actively pump calcium ions. Through SERCA, calcium ions are taken up, e.g., from the cytosol into the smooth ER lumen. SERCA is also the one that it uses to actively transport calcium ions to organelles at contact sites, e.g., into the Golgi lumen, for use in protein modification, sorting, and vesicular trafficking.
Calcium ion transport by the sarcoplasmic reticulum
As pointed out earlier the sarcoplasmic reticulum, a specialized type of smooth ER, plays a key role in the release (and storage) of calcium ions in the muscle cells.
Read on the infobox below for an overview of the various parts or regions in the sarcoplasmic reticulum and the muscle cell.
NOTE IT!
“Have you noticed how the Sarcoplasmic Reticulum is highly specialized in different muscle cells?”
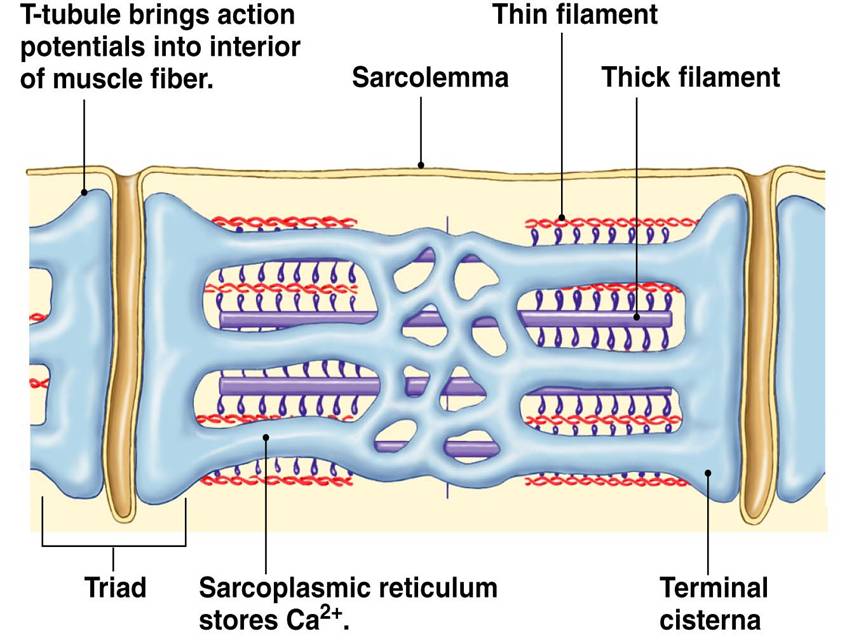
In skeletal muscle cells…
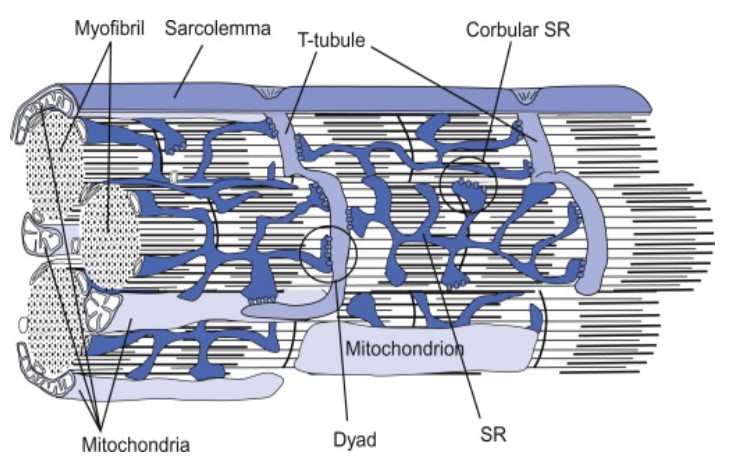
» The sarcoplasmic reticulum wraps around the myofibril, similar to that white foam net wrapped around a fruit. There are two regions: the longitudinal SR (runs parallel to the myofibrils) and the terminal cisternae.
» The terminal cisternae are the enlarged ends of the sarcoplasmic reticulum that serve as the primary Ca2+ reservoir.
» The holes in the sarcolemma (the cell membrane of the muscle cell) lead to the transverse tubules — where each tubule connects the invaginations formed between the sarcolemma and the sarcoplasmic reticulum.
» One transverse tubule and two adjacent terminal cisternae form the so-called “triad”. It is in this region that the Ca2+ ions are primarily released and absorbed by the sarcoplasmic reticulum.
In the smooth and cardiac muscle cells…
The sarcoplasmic reticulum of the smooth muscles and the cardiac muscles, though, are organized or arranged differently. While the sarcoplasmic reticula in the skeletal muscles are well-organized as shown above, the ones in the smooth muscle cells and in the cardiac muscle cells seem to be less organized.
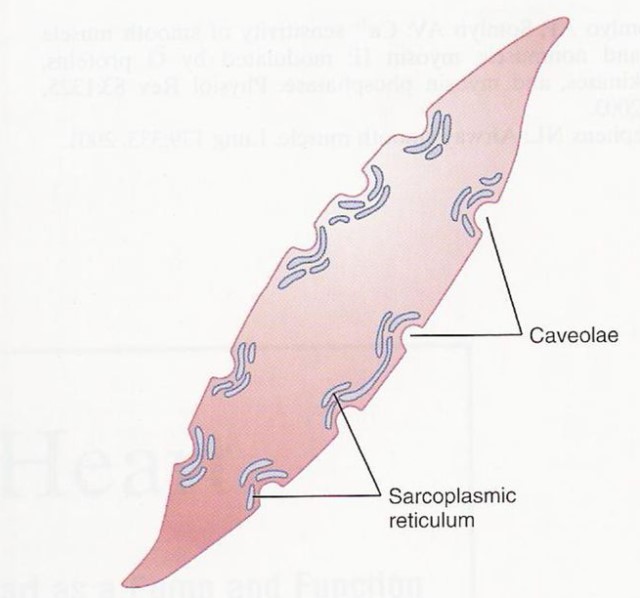 |  |
| Figure 8: (1) The sarcoplasmic reticulum of some smooth muscle cells. Notice the caveolae, which are the rudimentary analog of skeletal muscles’ T-tubules. Image Credit: Brainkart.com (Left) (2) The sarcoplasmic reticulum of cardiac muscle cells. Notice how a dyad is formed when the sarcoplasmic reticulum is in junction with the T tubule (vs. the triad in a skeletal muscle cell). Image Credit: Feher, J. (2012) (Right) | |
» In a skeletal muscle cell…
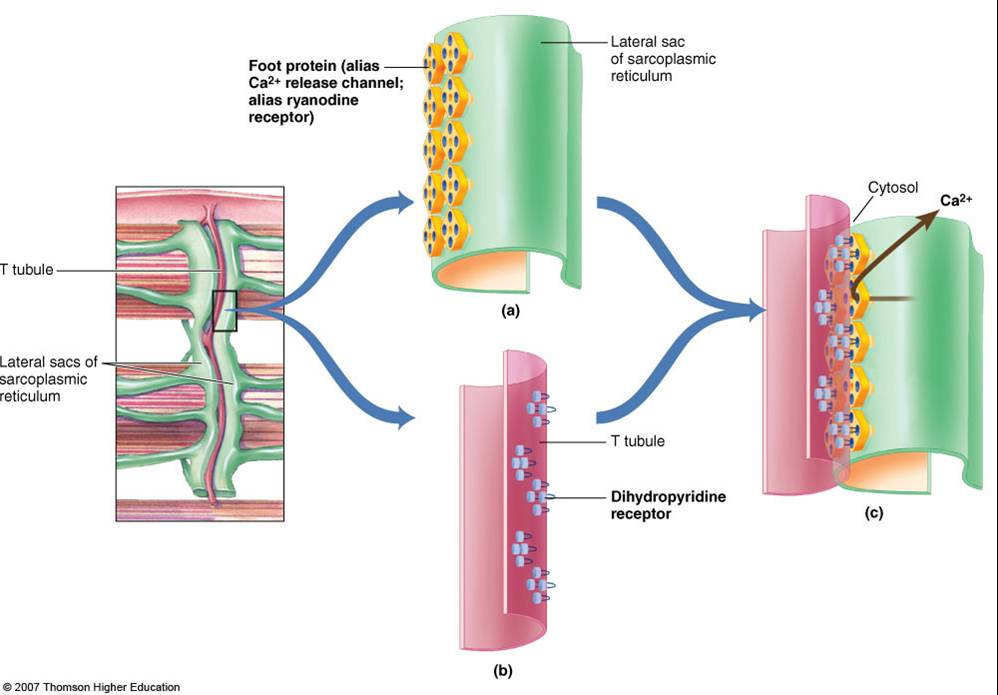
The calcium ion release occurs as follows. The excitation of the muscle cell results in an action potential that propagates along the sarcolemma deep into the muscle fiber via the T-tubules. The voltage-sensitive proteins in the T-tubule (called dihydropyridine receptors) are activated. They, then, activate the calcium channels (called ryanodine receptors, RyRs) in the terminal cisternae (major sites of Ca2+ ions storage of sarcoplasmic reticulum) to open. Ca2+ ions are released into the sarcoplasm. This triggers a positive feedback loop (called calcium-induced calcium release, CICR) wherein the neighboring RyRs are also triggered to open, amplifying the release of Ca2+ ions.
Ca2+ ions bind to the troponin. This results in the pulling of the tropomyosin, changing its position. This exposes the actin-binding sites. The myosin head gains access, causing it to bind to actin. Using energy by losing a phosphate group from the ATP, the myosin head swivels and produces a power stroke that pulls the thin filament inward, ultimately resulting in contraction.

Relaxation of the muscle fiber relaxes entails the uptake of Ca2+ ions back to the lumen of the sarcoplasmic reticulum via the Ca2+-ATPase pump in the terminal cisternae.
» In cardiac muscle cells and smooth muscle cells…
In the cardiac muscle cells, the process entails the excitation of the cardiac muscle cell first. This results in the generation of an action potential that propagates along the cell membrane deep into the muscle cell via the T-tubules. This leads to the opening of the L-type calcium channels (LTCCs) present in the T-tubule membrane, resulting in the influx of Ca2+ ions. This triggers the release of Ca2+ ions into the cytosol via the activated RyRs of the sarcoplasmic reticulum at the dyad.
In smooth muscle cells, the general steps include the activation of the receptors on the cell membrane of the smooth muscle cell, e.g., G-protein coupled receptors (GPCRs). Their activation leads to a cascade of reactions, such as the activation of phospholipase C that hydrolyzes phosphatidylinositol 4,5-bisphosphate into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. IP3, in particular, binds to the IP3Rs present in the membrane of the sarcoplasmic reticulum. This triggers the release of Ca2+ ions into the cytosol. They, then, bind to calmodulin and activate the myosin light chain kinase (MLCK), which ultimately leads to smooth muscle contraction.
Glucose metabolism
The ER participates in glucose metabolism and homeostasis through the enzyme glucose-6-phosphatase located in the ER membrane. This enzyme catalyzes the final step in gluconeogenesis and glycogenolysis by converting glucose-6-phosphate into glucose near the ER membrane facing the lumen. It does so by removing the phosphate group from the glucose-6-phosphate, generating glucose as a product.
For this to happen, the ER actively takes up glucose-6-phosphate from the cytosol into the ER lumen most likely by membrane transporters, such as glucose-6-phosphate translocase, an antiporter (i.e., simultaneously exporting inorganic phosphate out of ER in the process), with the expenditure of ATP energy.
Glucose exits the smooth ER lumen passively across the membrane via the glucose transmembrane carrier protein, GLUT2 (a rather ubiquitous bidirectional transporter of glucose in biological membranes that facilitates the diffusion of glucose, depending on the concentration of glucose).
Summary of calcium ion dynamics via the smooth ER
Below are the general steps of calcium ion uptake, release, and reuptake via the smooth ER.
1 Calcium uptake and storage is done by pumping Ca2+ ions from the cytosol into the lumen of the smooth ER through its calcium ATPases (e.g., SERCA pumps). The active transport of Ca2+ ions from the cytosol to the lumen uses ATP the amount of Ca2+ ions would therefore be higher in the smooth ER lumen than in the cytosol.
2 Ca2+ ions are released through activated calcium channels, such as IP3Rs or RyRs, in response to specific stimuli. In the cytosol, Ca2+ ions serve as signaling molecules to mediate distinct biological processes or to initiate specific cellular responses.
3 Ca2+ ions are taken up again, e.g., by SERCA pumps of the smooth ER. These pumps use ATP energy to actively transport Ca2+ ions back into the lumen for storage and future use.
Organellar and substructural formations
The endoplasmic reticulum contributes to the formation of organelles and other cellular structures, particularly, by supplying membrane components for membrane-bound organelles, such as the Golgi apparatus and the mitochondria.
The endoplasmic reticulum may also serve as a source for transport vesicles. In particular, the transitional ER forms a transport vesicle containing the newly synthesized protein. The vesicle buds off and travels along the microtubules toward its target, e.g., the Golgi apparatus. By fusing with the Golgi membrane, its “cargo” is transported into the Golgi lumen. The nascent protein will then be further modified and sorted and packaged into another transport vesicle that buds off from the Golgi so as to reach its final destination (within the cell or outside the cell).
ER Stress response
Should there be an imbalance between the protein folding capacity and the protein load in the ER, endoplasmic stress (ER stress) response may occur. This response is also referred to as the unfolded protein response (UPR response). This happens during certain disturbances that occur in the ER, e.g., dysfunction in redox regulation, dysfunction in calcium regulation, viral infection, glucose deprivation, and the accumulation of unfolded proteins or misfolded proteins.
There are three main signaling pathways that monitor protein folding and detect misfolded or unfolded proteins. These are essentially protein sensors embedded in the ER membranes:
- ATF6 (activating transcription factor 6)
- PERK (protein kinase R (PKR)-like kinase)
- IRE1 (inositol requiring enzyme 1).
A distinctive feature of a misfolded protein is the lack of glucose residues. When these sensors are activated, an ER stress response is triggered.
» The initial response is for glycosylation by the enzyme UGGT (UDP-glucose: glycoprotein glucosyltransferase).
» A heat shock protein (glucose-regulated protein 78) binds to the hydrophobic residues of the misfolded protein to prevent its transit.
» If protein misfolding continues, the protein is headed toward degradation to prevent it from aggregating with other misfolded proteins.
» By endoplasmic reticulum-associated degradation (ERAD), the ERAD chaperone shuttles the misfolded protein to the cytosol for degradation by cytosolic proteasomes (via the ubiquitin-proteasome pathway).
» If these measures fail to restore the normal function of the cell within a certain period of time, the next response is geared towards apoptosis.
Failure to regulate the UPR is implicated in the manifestation of certain diseases, such as metabolic diseases, neurodegenerative disorders, and cancer. This highlights the importance of increasing the ER’s protein folding capacity by reducing the burden of misfolded proteins, thereby, promoting cell survival.
Endoplasmic Reticulum Location
Where is the endoplasmic reticulum found inside the cell?
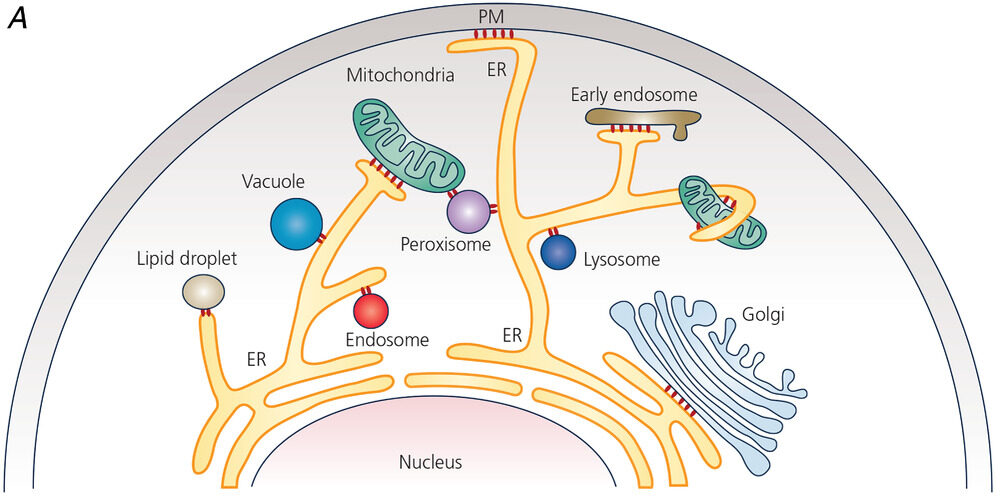
The endoplasmic reticulum is located in the cytoplasm of a eukaryotic cell. Oftentimes, it is found near the nucleus. However, the ER may extend to various parts of the cell and establish contact sites with other organelles and the plasma membrane. As discussed above, these contact sites are named depending on which the ER is associated with, such as the “ER-plasma membrane contact site” that connects the ER with the plasma membrane. See Figure 6.
Which types of cells have active endoplasmic reticulum?
Many cell types have rough ER that are primarily active in protein synthesis. Some of them are as follows:
- Mucus-secreting cells of the respiratory and the gastrointestinal tracts
- Plasma cells of the immune system, for the production and secretion of antibodies (immunoglobulins)
- Pancreatic cells, such as the exocrine cells, are secretory cells that produce and secrete digestive enzymes
- Hepatocytes, for the production and secretion of plasma proteins, such as albumin and clotting factors
- Cells of the adrenal cortex, for the production and secretion of steroid hormones (e.g., cortisol and aldosterone)
As for cell types with highly active smooth ER, some of them are:
- Muscle cells, for regulating calcium ion release and uptake essential in muscle contraction/relaxation
- Hepatocytes, for drug detoxification and metabolism
- Gonad cells (e.g. testes and ovaries), for the production of cholesterol and steroid hormones
- Cells of sebaceous glands, for the production and secretion of sebum (an oily substance that moisturizes skin and hair)
- Adipocytes, for storing and mobilizing lipids
And, of course, the muscle cells containing sarcoplasmic reticulum, which is a special type of smooth ER.
Note it! The type of ER may vary within cells…
Acell would have a greater rough ER when the cell is producing a large amount of protein. Conversely, smooth ER will be more pronounced if the cell is involved in producing more lipids.
Cell types with little or no endoplasmic reticulum
While there are multifarious cells that have prominent or abundant endoplasmic reticulum, there are cells that have scanty or no endoplasmic reticulum at all. Examples of mammalian cells with little or no ER:
- Red blood cells, not only do they have no ER, but these cells also lose their nucleus at maturity. They are otherwise specialized to carry oxygen more efficiently.
- Platelets are cells specialized for blood clotting. They have no ER and nucleus, too.
- Sperm cells are specialized for motility and fertilizing the ovum. Having a nucleus (containing the genetic material) in its “head” and mitochondria (for generating ATP) in its “neck”, a sperm cell does not seem to need too many other organelles like ER.
Note it!
“What happens to the endoplasmic reticulum during cell division?”
During cell division, especially prophase, the cell seems to have lost much of its subcellular contents, including organelles, as the chromosomes condense and become more palpable than the rest. But in reality, the organelles do not disappear like magic; rather, they temporarily disintegrate to varying degrees. The endoplasmic reticulum, in particular, partially disintegrates by fragmenting into smaller tubules and vesicles that are dispersed throughout the cytoplasm. Fragmentation entails various cellular events such as mitotic phosphorylation and interactions with the cytoskeletal elements. When the cell division is about to end, the ER fragments are distributed to each newly formed cell. Each cell would then have a share of the ER network that can reform and reorganize, with the potential to carry out its functions.
Table 1: Rough Endoplasmic Reticulum vs. Smooth Endoplasmic Reticulum | ||
|---|---|---|
| Features | Rough Endoplasmic Reticulum | Smooth Endoplasmic Reticulum |
| Morphology | A region of the ER consisting of an interconnected network of flattened sacs that are stacked on each other ( cisternae). The sheet-like structure offers a larger surface area for ribosomes during protein synthesis | A region of the ER consisting of tubules and vesicles. The tubular shape helps to increase surface area (and is well-suited) for lipid synthesis and detoxification |
| Presence of Ribosomes | Ribosomes dock to the ribosome receptor present in the rough ER membrane during protein synthesis (as escorted by the signal recognition particle) | Ribosomes do not typically attach to it but do so under rare conditions, especially cellular stress. Ribosomes attach to the smooth ER in hepatocytes, for instance, during starvation-induce autophagy. |
| Function | » Primarily, protein synthesis and protein folding. Some of the common synthesized proteins are:
» Protein quality control (e.g., by UPR sensors, chaperones, and lectins that monitor and recognize misfolded proteins) » Intracellular transport, e.g., the shuttling of a newly formed protein destined to the Golgi apparatus for further maturation. While N-linked glycosylation occurs in the rough ER, O-glycosylation occurs in the Golgi.
| » Lipids synthesis, metabolism, and trafficking (e.g. phospholipid synthesis for integration to the lipid bilayer of biological membranes) » Intracellular calcium ion dynamics and homeostasis (via calcium ion channels, such as inositol trisphosphate receptors, Sarco/Endoplasmic Reticulum Calcium ATPase, and ryanodine receptors) for various cellular functions, including muscle contraction » Drug detoxification (via cytochrome P450 enzymes and UDP-glucuronosyltransferases) » Glucose metabolism (i.e. by converting glucose-6-phosphate to glucose during gluconeogenesis or glycogenolysis via the sER membrane enzyme glucose-6-phosphatase) » Intracellular transport, e.g., lipid trafficking through membrane contact sites
|
| Membrane Structure | Has a single membrane, with typical markers, such as ribosomes and transmembrane protein ribophorin I involved in protein synthesis, particularly, during the translocation of the nascent polypeptide chain | Has a single membrane, with cytochrome P450 enzymes as the typical markers used by researchers to distinguish the smooth ER from the rough ER |
| Luminal content | Primarily, proteins involved in protein translocation, protein folding, post-translational modifications, protein quality transport, and initial stages of transport (e.g., enzymes, secretory proteins, and chaperone proteins) | Luminal contents vary depending on the nature and function of the smooth ER: Enzymes that mediate lipid synthesis (e.g., acyl-CoA synthetases, acyltransferases, phosphatidate phosphatase, diacylglycerol acyltransferase, and phosphatidylserine synthase) Enzymes such as cytochrome P450s for drug detoxification Calcium ions |
| Special Types | – | Sarcoplasmic reticulum |
| Location in the cell | In the cytoplasm, typically depicted near the nucleus as it is associated with protein synthesis although may extend as it establishes contact sites with other subcellular components | In the cytoplasm, typically depicted near the Golgi apparatus although may extend as it establishes contact sites with other subcellular components |
| Cell types typically found or most abundant |
|
|
Data summarized by Maria Victoria Gonzaga for Biology Online
Take the Endoplasmic Reticulum – Biology Quiz!
Further Reading
- Role of Golgi Apparatus & Endoplasmic Reticulum in Protein Synthesis
- Protein Synthesis – Tutorial
- Biology Forum – Where does protein synthesis take place
References
- Claude, A., Fullam, E. F., & McLean, J. R. (1945). The characterization of a granulated cytoplasmic component (G-bodies) and its relation to microsomes. Journal of Experimental Medicine, 81(2), 185-197.
- CK-12 Foundation. (2023). CK12-Foundation. CK-12 Foundation; CK-12 Foundation. https://flexbooks.ck12.org/cbook/ck-12-biology-flexbook-2.0/section/2.7/primary/lesson/ribosomes-and-mitochondria-bio/
- Vance, D. E. (1990). The biochemistry of lipids: the composition of cell membranes. Science, 250(4988), 766-772. doi: 10.1126/science.2237410
- Cytochrome P450 enzymes in the rough endoplasmic reticulum:
Guengerich, F. P. (2001). Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chemical Research in Toxicology, 14(6), 611-650. doi: 10.1021/tx0002583 - Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2005). Principles of biochemistry (4th ed.). W. H. Freeman.
- Hetz, C., & Saxena, S. (2017). ER stress and the unfolded protein response in neurodegeneration. Nature Reviews. Neurology, 13(9), 477–491. https://doi.org/10.1038/nrneurol.2017.99
- Zhang, J., Wang, J., Xu, J., Lu, Y., Jiang, J., Chen, Y., & Wang, X. (2016). Endoplasmic reticulum-resident protein 29 (ERp29) is involved in the formation of autophagosomes through the interaction with ATG14 and promoting LC3 lipidation. Autophagy, 12(8), 1488-1499. doi: 10.1080/15548627.2016.1190061
- Hoozemans, J. J. M., Veerhuis, R., Van Haastert, E. S., Rozemuller, A. J. M., Baas, F., & Eikelenboom, P. (2007). The unfolded protein response is activated in Alzheimer’s disease. Acta neuropathologica, 114(3), 279-287.
- Liu, Y., Ge, L., Ma, J., Li, N., & Li, K. (2019). Therapeutic potential of targeting the endoplasmic reticulum stress response pathways in Alzheimer’s disease. Frontiers in pharmacology, 10, 682.
- Associate Degree Nursing Physiology Review. (2023). Austincc.edu. https://www.austincc.edu/apreview/PhysText/Muscle.html
- Lecture:27 Protein Targeting- Endoplasmic Reticuluum. (2023). Zoology.ubc.ca. https://www.zoology.ubc.ca/~berger/B200sample/unit_8_protein_processing/er_targeting/lect27.htm
- Nebert, D.W., et al. (2013). Drug Metabolism Enzymes in the Superfamily Cosmetics, and Pharmaceutics. In Comprehensive Toxicology (Third Edition), 2013. DOI: 10.1016/B978-0-12-387815-1.00311-2.
- Anzenbacher, P., and Anzenbacherová, E. (2001). Cytochromes P450 and metabolism of xenobiotics. Cell and Molecular Life Sciences, 58(5-6), 737-747. DOI: 10.1007/PL00000897.
- Hayes, A.W. (2014). Principles and Methods of Toxicology (Sixth Edition). CRC Press. ISBN: 978-1-4822-4019-0.
- Associate Degree Nursing Physiology Review. (2023). Austincc.edu. https://www.austincc.edu/apreview/PhysText/Muscle.html
- Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2014). Molecular Biology of the Cell (6th ed.). Garland Science.
© Biology Online. Content provided and moderated by Biology Online Editors