Reviewed by: Todd Smith, PhD
Definition
noun, plural: energies
(1) The capacity for work.
(2) The ability to do work, or produce change.
Supplement
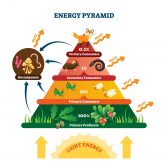
Energy exists in different forms but is neither created nor destroyed; it simply converts to another form. Examples of energy include kinetic, potential, thermal, gravitational, elastic, electromagnetic, chemical, nuclear, and mass. Energy can be expressed in joules or ergs.
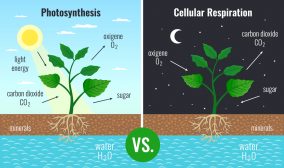
In biology, energy is often stored by cells in biomolecules, particularly carbohydrates (sugars) and lipids. Energy is released during the formation of chemical bonds (Baird, 2013), such as during the redox reactions of cellular aerobic respiration.
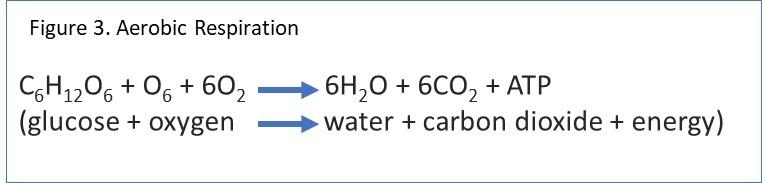
The chemical formula depicting the energy released when the chemical bonds in sugar are broken-down during the aerobic respiration is a simplification of that chemical reaction:
glucose (sugar molecule) + oxygen –> water + carbon dioxide + energy
This chemical reaction, if expanded, shall entail two distinct events, particularly the breaking of the original chemical bond and the subsequent formation of a new bond. The above chemical reaction may not show it but an energy input is required to break the chemical bonds in the substrate. Energy is released when a new and strong chemical bond has formed.
Why does the chemical reaction above show energy only as a product? Aerobic respiration is an exothermic process, which means the energy released from new bond formations is greater than the energy needed to break the original bonds. The net energy is positive, which also means there is a net release of energy. (Baird, 2013)
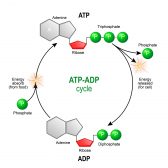
The released energy may then be carried by an energy-carrier molecule (ATP). And as such, the chemical reaction is also represented as follows:
Word origin: From Ancient Greek ἐνέργεια (energeia) “action, act, work”, < ἐνεργός (energos) “active” < ἐν (en) “in” + ἔργον (ergon) “work”.
Related forms: energize (verb), energetic (adjective).
Related phrases: kinetic energy, potential energy, solar energy.
References
Baird, C.S. (2013). When does the breaking of chemical bonds release energy? Science Questions with Surprising Answers. https://www.wtamu.edu/~cbaird/sq/2013/06/27/when-does-the-breaking-of-chemical-bonds-release-energy/