Hydrogen
n., ˈhaɪdɹədʒ(ə)n
Definition: A colorless, odorless, gaseous element, represented by the symbol “H”
Table of Contents
Hydrogen is one of the chemical elements found in nature. A chemical element refers to the pure substance of one type of atom. At present, 94 are natural elements whereas 24 are synthetic. Hydrogen is one of the most common elements in living things, together with carbon, oxygen, and nitrogen. It is also the second-most-abundant element in the universe, after helium.
Hydrogen Definition
Properties of Hydrogen
Hydrogen is a natural gaseous element with an atomic number of 1. It is regarded as the lightest element with its atomic weight of 1.0079. As gas, it is one of the few chemical elements that are stable diatomic homonuclear molecules at STP.
Hydrogen is one of the reactive nonmetals and has an electron configuration of 1s1. It combines with oxygen to form water (H2O) and is present in all organic compounds. Hydrogen gas itself is not poisonous, but when it mixes with air it can easily ignite or explode. The melting point of oxygen is -259.16°C. Its density at STP is 0.08988 g/L.
In the 18th and 19th centuries, scientists learned that the air components could be liquefied by compressing and cooling the air. Hydrogen can be liquefied fully by cooling it below the critical point of 20.28 K. Solid hydrogen is the solid-state of hydrogen. It can be formed by decreasing the temperature below −259.14 °C. Slush hydrogen is a combination of liquid and solid hydrogen.
Hydrogen Ions
Hydrogen ion forms when the hydrogen atom loses or gains an electron. In particular, the loss of electron in a hydrogen atom produces hydrogen cation (H+) whereas the gain of the electron produces hydrogen anion H–.
Hydrogen cations include the following:
- hydron (H+)
- proton (1H+) – the cation of the isotope protium
- deuteron (2H+) – the cation of the isotope deuteron
- triton (3H+) – the cation of the isotope tritium
- hydronium ion (H3O+) – an aqueous hydrogen cation
Hydrogen anions are exemplified by the following:
- hydride (H–)
- protide (1H–) – the anion of the isotope protium
- deuteride (2H–) – the anion of the isotope deuteron
- tritide (3H–) – the anion of the isotope tritium
Isotopes of Hydrogen
The most common naturally-occurring isotopes of hydrogen are Hydrogen-1 (protium), Hydrogen-2 (deuterium), and Hydrogen-3 (tritium). Hydrogen-1 has one proton but no neutrons in its nucleus. It is a stable and the most abundant naturally-occurring isotope of hydrogen. Its natural abundance (NA, i.e. the abundance of the isotope in nature) is 99.985%. Hydrogen-2 is also a stable isotope. Its nucleus, called deuteron, has one proton and one neutron. Its NA in the oceans is 0.0115%. Hydrogen-3 is a radioactive isotope. It has two neutrons and one proton. Unlike Hydrogen-1 and Hydrogen-2, Hydrogen-3 is not stable. It emits beta-particles to become stable. Thus, it is used in some glow-in-the-dark paints and as a tracer in biological studies.
Allotropes of Hydrogen
An allotrope of an element pertains to any of the multiple substances formed by only one type of element. Examples of allotropes of hydrogen are atomic hydrogen and diatomic hydrogen.
1. Atomic hydrogen
Atomic hydrogen refers to the isolated hydrogen atoms, which are extremely rare in nature. Hydrogen atoms are mostly found interacting with other atoms in compounds, such as in water and organic compounds.
2. Diatomic hydrogen
Diatomic hydrogen has a formula of H2. It is also called hydrogen gas. It is a stable molecule. It consists of two hydrogen atoms bound together (thus, the name). They share electrons, forming a covalent bond. This allotrope is colorless, odorless, tasteless, and highly combustible at standard temperature and pressure.
Spin Isomers of Hydrogen
There are two spin isomers of hydrogen: orthohydrogen and parahydrogen. The orthohydrogen is a spin isomer of hydrogen wherein the two proton spins parallel whereas the parahydrogen is one in which the two proton spins antiparallel.
Hydrogen Compounds
A hydride pertains to the anion of the hydrogen, i.e. H–. It may also refer to a compound where hydrogen reacts with a more electropositive element or groups, forming a compound. Also, hydrogen that is bound to metals or metalloid is also called hydrides. Some of the common natural hydrides are ammonia (NH3), ethane (C2H6), and methane (CH4).
Inorganic compounds are those substances that generally lack Carbon-Carbon (C-C) and Carbon-Hydrogen (C-H) bonds. Examples of biologically important inorganic compounds containing hydrogen are water (H2O) and hydrochloric acid (HCl, which is produced by the stomach).
Organic compounds are fundamentally defined as those substances containing carbon atoms and Carbon-Carbon (C-C) and Carbon-Hydrogen (C-H) bonds. Hydrocarbons are one of the chief organic compounds. They contain both carbon and hydrogen.
Discovery of Hydrogen
In 1766, British chemist, Henry Cavendish 1731 –1810, discovered hydrogen. He called the gas inflammable air and described it in his paper, “On Factitious Airs”. He was not the first to have prepared hydrogen gas. Robert Boyle 1627 –1691, an Anglo-Irish chemist and regarded as the first modern chemist, was one of those who had done it earlier. However, Cavendish was often the one credited for discovering hydrogen for correctly assuming its elemental nature.
In 1783, French chemist Antoine Lavoisier 1743 –1794 gave the name hydrogen. (He was also credited as the one who gave the name oxygène in 1777, which was later adopted into the English oxygen). The English name hydrogen was derived from the Greek ὑδρο (hydro, meaning “water”) and -γενής (-genēs, meaning “producer”).
Cavendish found that the inflammable air (now, hydrogen), when reacted with dephlogisticated air, produces water. He reported his findings to Joseph Priestly, who in turn was credited to have discovered dephlogisticated air (now, called oxygen). In 1784, Cavendish presented his findings to the Royal Society. He elucidated that water was a compound comprised of inflammable air (hydrogen) and dephlogisticated air (oxygen) and that the proportion was two is to one.
Biological Importance
In biology, hydrogen plays a crucial role in various biochemical and physiological processes. It is the third most abundant element (9.5% by mass) in the human body, after oxygen (65%) and carbon (18.5%).
Proton gradient
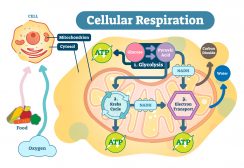
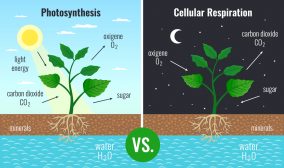
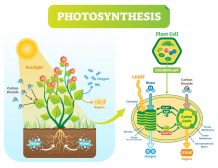
The proton gradient (i.e. the gradient of hydrogen ions) is essential to organisms. For instance, the gradient can drive ATP synthase, a membrane enzyme complex that catalyzes ATP synthesis. The enzyme has a channel portion by which H+ can diffuse through. As they move across, the enzyme undergoes a conformational change that brings the ADP molecule and inorganic phosphate together, forming ATP. In mitochondria and chloroplasts, this proton gradient is further used to drive the release of ATP produced from oxidative phosphorylation and from photophosphorylation, respectively.1

Ionized hydrogen
Ionized hydrogen is chemically reactive. H+ tends to react readily with other atoms or molecules with electrons. Molecules or atoms that accept H+ are called bases whereas those that donate H+ are called acids. pH measures the hydrogen ion concentration of a solution. pH level that is below 7 indicates acidity whereas a pH level above 7 indicates alkalinity.
The pH of different cellular compartments, body fluids, and organs is usually tightly regulated in a process called acid-base homeostasis. Lysosomes have a pH level of 4.5. Cytosol has a neutral pH, i.e. 7.2. The mitochondrial matrix has a pH of 7.5. The human gastric acid has a pH ranging from 1.5 to 3.5. Human skin has a pH of 4.7. Urine has a pH o 6.0. Blood has a pH between 7.34 and 7.45. Pancreatic secretions have a pH o 8.1.
In microorganisms, pH is an essential factor in their growth and survival. Microorganisms that prefer an acidic environment are called acidophiles whereas those that prefer an alkaline environment are called alkaliphiles. And, microorganisms that thrive in a neutral environment, i.e. neither acidic nor alkaline, are called neutrophiles.
Hydrogen bonds
In organic compounds, a hydrogen bond is important in providing stability of many biomolecules. The hydrogen bond is also the bond that glues water molecules together. Hydrogen bond formation in water accounts for the latter’s distinctive properties such as high boiling point (100 °C), high surface tension, specific heat, and heat of vaporization.2
The hydrogen bond is also essential to organisms as it is responsible for the formation of important molecules. It occurs in inorganic molecules (e.g. water) and organic molecules (e.g. DNA and proteins). Hydrogen bonds are responsible for the secondary and tertiary structures of important biomolecules such as nucleic acids and proteins. Hydrogen bond has a structural role during the formation of polymers, both synthetic (e.g. nylon) and natural (e.g. cellulose).
READ: What is Hydrogen Bond – Definition and Examples
Hydrogen peroxide
In humans and other vertebrates, hydrogen peroxide (H2O2) produced in phagocytes is used to destroy pathogens. In plants, hydrogen peroxide is released in response to the presence of a fungal attack.
READ: Plant Cell Defense – Biology Tutorials
Hydrogen Cycle
Hydrogen is the most abundant element in the universe followed by helium. On Earth, it is also a chief element that is being cycled biologically and geologically as depicted in the so-called hydrogen cycle. However, unlike in other biogeochemical cycles, hydrogen, being a low molecular weight element, has a tendency to leave the Earth’s atmosphere.
The sources of hydrogen include methane and non-methane hydrocarbon oxidation, nitrogen fixation, oceans, biomass burning, fossil fuel, and industrial output. Biologically, H2 is produced by certain microorganisms via certain types of anaerobic metabolism, e.g. reactions catalyzed by hydrogenases, i.e. enzymes that oxidize hydrogen (removing its electrons) and then attach it to another molecule. Interspecies hydrogen transfer occurs in the symbiosis between methanogens (methane-producing archaea) and non-methanogenic anaerobes. The non-methanogenic anaerobes produce and release H2 by metabolizing organic substances. Methanogens, in turn, take up H2 and convert it into methane.
Biological activities are the predominant sink for H2) from the atmosphere. The non-biological sink for H2) involves hydroxyl radicals. H2 interacts with hydroxyl radicals (OH), forming a water (H2O) molecule.
READ: The Water Cycle – Biology Tutorials
Derived terms
- Heavy hydrogen
- Hydrogen Acceptor
- Hydrogen bond
- Hydrogen Carrier System
- Hydrogen peroxide
- Hydrogen sulfide
- Hydrogen-3
- Hydrogen-transporting ATP synthase
- Sodium hydrogen carbonate
See also
- element
- proton gradient
- hydrogen bond
- pH
- Water
Reference
- Berg, J. M., Tymoczko, J. L., & Lubert Stryer. (2002, January 1). A Proton Gradient Powers the Synthesis of ATP. Retrieved from ://www.ncbi.nlm.nih.gov/books/NBK22388/ Link
- Rae-Dupree, J. & DuPree, P. (2019, January 1). 4 Types of Chemical Bonds – dummies. Retrieved from ://www.dummies.com/education/science/anatomy/4-types-of-chemical-bonds/ Link
© Biology Online. Content provided and moderated by Biology Online Editors.