Definition
noun
(chemistry)
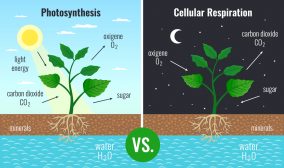
(1) A chemical reaction in which the interaction of a compound with water results in the decomposition of that compound.
(2) A chemical reaction wherein a water molecule (HOH) and a reactant exchange functional groups resulting in two end products, one containing the hydrogen cation (H) and the other the hydroxyl anion (OH). An example is the chemical reaction of water with the ions of salt resulting in the formation of an acid and a base, one or both of which is only slightly dissociated.
(3) The process of splitting a compound into fragments with the addition of water; a kind of reaction that is used to break down polymers into simpler units, e.g. starch into glucose.
Supplement
Word origin: Gk. hydor = water + lyein = to loosen, dissolve.
Related forms: hydrolytic (adjective), hydrolyze (verb).
See also: water.
Dictionary > Hydrolysis