
Hydrophilic
adj.
/ˈhaɪdrəʊ.fɪlɪk/Definition: Capable of interacting with water through hydrogen bonding
Table of Contents
Hydrophilic Definition
What does a hydrophile (or hydrophilic molecule) mean? If a molecule is “water-loving”, it is known as ‘hydrophile’ (noun) that possesses a hydrophilic nature. In contrast, if a molecule doesn’t like water i.e. repel water, it is known as ‘hydrophobic‘. The terms hydrophilic and hydrophobic are used to describe molecules or substances based on how they react to water molecules. The degree or extent to which a molecule or surface attracts water is known as the ‘hydrophilicity‘ of that molecule. Some of the most common examples of hydrophilic substances are sugar, salt, starch, and cellulose.
Hydrophilic means water-loving; having an affinity for water; capable of interacting with water through hydrogen bonding. Etymology: from Greek hydros, meaning “water” and philia, meaning “friendship”. Compare: hydrophobic
Hydrophilic substances are polar in nature. ‘Like dissolves like’ theory governs the fact that hydrophilic substances tend to readily dissolve in water or polar solvents while hydrophobic substances are poorly soluble in water or polar solvents.
We all have seen the example of hydrophilic substances in our daily lives. Each one of us has seen that sometimes water spread out evenly on a surface while in certain cases it forms small droplets.
Why so?
It’s because certain surfaces are water-loving or hydrophilic and hence water spreads out while in the case of poorly hydrophilic substances (or hydrophobic substances), it forms tiny droplets as these surfaces repel water.
Let’s continue reading the next sections to learn what else defines a hydrophilic molecule.
Chemistry Behind Hydrophilicity
Hydrophilic molecules or Hydrophilic moieties are basically polar compounds that have ionic groups. The polar nature of these hydrophilic molecules enables them to readily absorb water or polar solvent and eventually get dissolved in polar solvents like water. Being a polar protic solvent, water is capable of forming a hydrogen bond (-H—-OH-). Hydrophilic molecules are polar in nature and easily form a hydrogen bond with water thereby getting dissolved in water. Notably, these interactions between the hydrophilic molecule and water are thermodynamically favored. In general, hydrophilic substances can easily form hydrogen bonds with polar solvents like water, and alcohol.
Chemically, hydrophilic substances have ionic (charged) groups that contain oxygen or nitrogen atoms. The polarity of a substance usually defines its hydrophilicity. Some of the common functional groups found in hydrophilic substances/surfaces are enlisted in Table 1.
Table 1: Some of the common hydrophilic and hydrophobic functional groups
| Chemical groups in hydrophilic substances | Chemical groups in hydrophobic substances |
|---|---|
| -OH | -CH3 |
| -COO- | -CH2-CH3 |
| -NH- | -R-C6H5 |
| -Aln (OH)m, etc | C2H2, etc |
As a general rule, the hydrophilicity of any surface varies as per the functional group and ability for hydrogen bonding: non-polar < polar, no hydrogen bonding < polar, hydrogen bonding < hydroxylic, ionic. Hydrophilicity is significantly influenced by the number of sites and the structure and density of the interphase area.
Measurement of Hydrophilicity
Contact angle measurement is a major parameter to quantify the hydrophilicity of a substance, which is further indicative of wettability. Hydrophilic substances possess good wettability. Wettability is the ability of the liquid to remain in contact with the solid surface. The degree of wettability is measured using a contact angle. The contact angle (θ) is the angle between the surface and the edge of the droplet. A hydrophilic surface has a contact angle (θ) <90° while the hydrophobic surface exhibits a contact angle (θ) >90°, shown in Figure 1 (below). A higher contact angle indicates a stronger liquid-liquid interaction rather than liquid-surface interaction thus making the material hydrophobic.
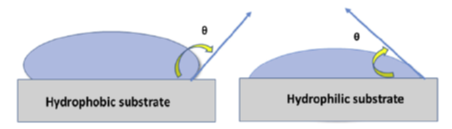
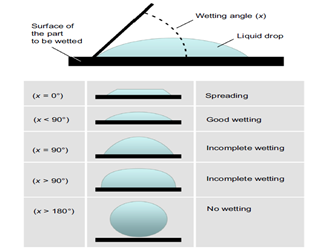
If the liquid spreads out on a surface, wetting a large area of the surface, then the contact angle is less than 90° and is considered hydrophilic, or water-loving (Figure 2). While, if a liquid forms a droplet, the contact angle is more than 90° and is considered to be hydrophobic or water-repelling (Figure 2). Wettability is an important parameter for plants and animals. Lotus flower leaves and Rice leaves exhibit a non-wetting surface, wherein leaves remain dry and water droplets roll out from the surface of the leaves keeping them clean all the time. Certain animals like Namib desert beetles, manage to survive in the dry region due to their ability to absorb moisture from the environment via hydrophilic structures on their body surface.
From the above discussion, we now know that hydrophilic surfaces tend to spread out the water over their surface and do not allow the formation of water droplets. This functionality of the hydrophilic surfaces is utilized to make anti-fogging surfaces in the automobile industry.
Due to its hydrophilic nature, a substance tends to possess water absorption ability via capillary action. The extent of water absorption of a hydrophilic substance depends on the porosity of the substance.
Applications of Hydrophilic Substances
Hydrophilic polymers and molecules are widely utilized in the field of physics, chemistry, engineering, biomedical, drug delivery, food, pharmaceuticals, paint, textiles, paper, constructions, adhesives, coatings, water treatment, dispersing and suspending agents, stabilizers, thickeners, gellants, flocculants and coagulants, film-formers, humectants, binders and lubricants, personal care, building products, detergents, oil field products, and mineral processing, etc.
Hydrophilic polymers exhibit good water vapor permeability due to ionic groups. Clothing or apparel that is required to be breathable is made up of hydrophilic fibers.
Hydrophilic polymers like Cellulose, Alginate, and chitosan are the most extensively used in the food industry wherein they are used as a thickening agent, stabilizer, and gelling agent.
Addition of hydrophilic substances like starch-based compounds to the home-grown plant pots. This helps to reduce the requirement of frequent watering and consumption.
Hydrophilic substances have the ability to absorb and hold water. Hydrogels are a type of hydrophilic polymer that are widely utilized in sanitary products, biomedical engineering, bioseparation, agriculture, food processing, and oil recovery, to mention a few. The characteristic property of these hydrogels is to absorb water and swell. Hydrophilic hydrogels also have a soft character along with biocompatibility. Hydrogels are copolymers or homopolymers that are prepared by crosslinking of monomers. These monomers have an ionizable group or a functional group that can be ionized. Hydrogels may contain weakly basic groups like substituted amines, weakly acidic groups like carboxylic acid, or strong basic and acidic groups like quaternary ammonium compounds and sulfonic acids. All these ionic groups make the hydrogels hydrophilic. Depending upon their ability to hold water/swelling, different hydrogels are utilized in different applications, for example, hydrophilic, non-porous, slow swelling hydrogel polymers are used in manufacturing contact lenses and artificial muscles, while, hydrophilic, microporous, fast swelling hydrogel polymers are used in making diapers. Polyacrylates and sodium polyacrylates are superabsorbent hydrophilic hydrogel polymers that are used in making diapers. These superabsorbent hydrogels can hold water equivalent to 100 times their own weight.
Hydrophilic hydrogels are similar to the extracellular matrix and for this reason, they are widely being explored for making artificial tissue scaffolds. Due to biocompatibility, hydrophilic hydrogels are widely used in biomedical applications. Gelatin is one of the widely used hydrophilic hydrogels. Gelatin is an animal by-product and is made up of protein & peptide-like, collagen. Gelatin is most commonly used for preparing capsules.
The hydrophilic hydrogel also helps to hasten the wound-healing process and hence they are widely used as a wound-healing agent.
Hydrophilic hydrogels are superabsorbent materials that are also widely used in drug-delivery systems, tissue repair, and cosmetics. Hydrophilic super porous hydrogels are used as disintegrants or super disintegrants in a tablet for achieving fast drug release from the tablet.
Hydrophilicity is a critical criterion for the absorption of a drug molecule. It is a well-established fact that for the absorption of a drug in the human body, the drug should be in a solubilized state. Hydrophilic drugs tend to easily dissolve and are solubilized, thereby enabling drug absorption. Thus, hydrophilic drugs having suitable permeability have a higher probability of getting absorbed in the body easily and exerting their therapeutic effects.
Hydrophilic substances are coated onto the surface of medical devices to reduce bacterial adhesion onto the surface of the medical device. Hydrophilic polymers like polyvinylpyrrolidone (PVP), polyurethanes, polyacrylic acid (PAA), polyethylene oxide (PEO), and polysaccharides are widely used as anti-fouling coatings on medical devices like catheters, stents. As soon as any medical device is placed in the body, the deposition of the protein layer is initiated. Over a period of time, this layer becomes very thick and can result in serious side effects viz., obstruction, etc. Hence, it is necessary to circumvent the formation of the protein layer on the surface of the medical device. Hydrophilic polymers act as an anti-fouling agent and thereby resist the build-up of this protein layer over the surface of the medical device. Additionally, these hydrophilic polymers help to reduce the coefficient of friction thereby enabling ease of installation of the medical device in the body.
For a similar reason, but in a different application, hydrophilic polymers or surfaces are used in parts of the marine structure that are used underwater. Due to compatibility with water, hydrophilic surfaces face reduced friction underwater, thereby aiding in their easy movement underwater.
Hydrophilic polymers are used as an anti-fouling agent on the filtration membranes in reverse osmosis (RO) filtration. Polymers like cross-linked poly (ethylene glycol) (PEG), triethylene glycol dimethyl ether (triglyme), Cellulose-based, etc are used in RO filtration membranes. Being hydrophilic in nature, these polymers allow the filtration of water through them and concurrently resist the development of a bacterial layer over them.
Fluoride acid treatment to dental implants is carried out to increase the hydrophilicity of the dental implants. This results in reduced healing time, the easy establishment of the implant, and also a firm anchoring of the implant.
There is a group of molecules that have both hydrophilic as well as a hydrophobic part in their structure, such molecules are known as Amphipathic molecules. The most common category of such molecules is commonly known as surfactants. However, the contribution or the size of the hydrophilic part and the hydrophobic part in a surfactant molecule determine its nature as ‘Hydrophilic moieties’ or ‘Hydrophobic moieties’. Depending on their nature, surfactant molecules are utilized in a wide variety of applications. A scale known as ‘Hydrophilic-Lipophilic Balance’ or HLB scale is used as guidance to understand the basic nature of surfactant molecules and use them accordingly. Thus, the HLB scale helps to understand the affinity of the surfactant molecule towards a solvent. In case the surfactant molecule exhibits a higher affinity towards the water or polar solvent, it is categorized under ‘Hydrophilic moieties’, while if a surfactant molecule exhibits a higher affinity towards non-polar or lipophilic solvents, it is categorized as hydrophobic or lipophilic. Surfactants are very important and critical for the formulation and stabilization of emulsions. The HLB scale was introduced by Griffin and usually ranges from 0-20. The categorization of surfactant molecules based on the HLB scale is enlisted in Table 2.
Table 2: HLB scale for the characterization of surfactants
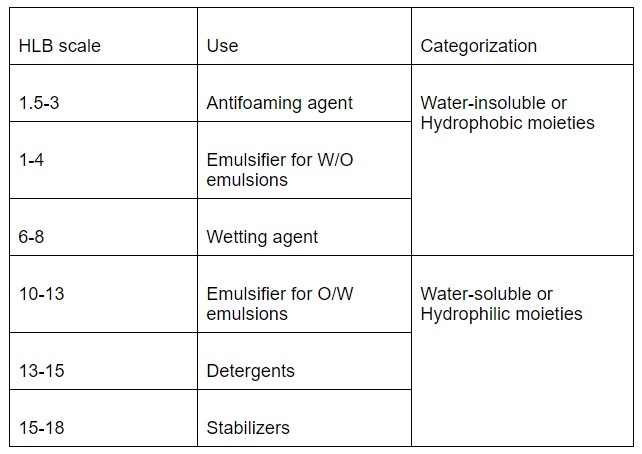
A lower HLB value is indicative of the water-repelling or hydrophobic nature of the surfactants while a higher HLB value is indicative of the water-loving or Hydrophilic nature of the surfactants. Propylene glycol monostearate, mono- and di-glycerides, lactylated monoglycerides, and succinylated monoglycerides are some of the few surfactants that fall under the category of hydrophobic or lipophilic surfactants, that have HLB less than 10 and can be used for the stabilization of W/O emulsions. Diacetyl tartaric acid esters of monoglyceride, polysorbates, and lecithin are some of the examples of hydrophilic surfactants and can be used for the stabilization of O/W emulsions. Interestingly, one of the most commonly used surfactants, Sodium lauryl sulfate has an HLB value of 40. These surfactants are widely used in the food and pharmaceutical industry.
Examples of Hydrophilic Substances
Some of the common examples of hydrophilic substances are as follows:
- Protein
- Keratin
- Wool
- Cotton
- Silica
- Gypsum
- Polyethylene glycol ethers
- Polyacrylic amide
- Polyurethanes with polyethylene glycol ether
- Polyvinyl alcohol (PVA)
- Polysaccharides (e.g. cellulose) and their derivatives (e.g. hydroxypropylmethylcellulose, hydroxyethylcellulose, and sodium carboxy methylcellulose)
- Gelatin, agar, agarose, algin
- Alcohols
- Cyclodextrins
- poly-N-vinylpyrrolidone (PVP)
- Guar gum, xanthan gum
- Starch
- Pectin
- Dextran
- Carrageenan
- Inulin
- Chitosan
- Albumin
Try to answer the quiz below to check what you have learned so far about hydrophilic.
READ: Chemical Composition of the Body – Biology Online Tutorial
References
- Ahmad D., van den Boogaert I., Miller J., Presswell R., Jouhara H. (2018). Hydrophilic and hydrophobic materials and their applications. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 40:22, 2686-2725, DOI: 10.1080/15567036.2018.1511642
- Erothu, H. and Kumar, A.C. (2016). Hydrophilic Polymers. In Biomedical Applications of Polymeric Materials and Composites (eds R. Francis and D. Sakthi Kumar). doi:10.1002/9783527690916.ch7
- Ismail A.F., Khulbe K.C., Matsuura T. (2019). RO Membrane Fouling, In: Reverse Osmosis, Ismail A.F., Khulbe K.C., Matsuura T. (Eds). Elsevier, pp. 189-220. doi.org/10.1016/B978-0-12-811468-1.00008-6.
- Law K. Y. (2014). Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. The Journal of Physical Chemistry Letters, 5(4), 686–688. https://doi.org/10.1021/jz402762h
- Ohshima, H., Yamashita, Y. and Sakamoto, K. (2016). Hydrophilic–Lipophilic Balance (HLB): Classical Indexation and Novel Indexation of Surfactant. In Encyclopedia of Biocolloid and Biointerface Science 2V Set, H. Ohshima (Ed.). doi:10.1002/9781119075691.ch45
- Piozzi, A., Francolini, I., Occhiaperti, L., Venditti, M., & Marconi, W. (2004). Antimicrobial activity of polyurethanes coated with antibiotics: a new approach to the realization of medical devices exempt from microbial colonization. International journal of pharmaceutics, 280(1-2), 173–183. https://doi.org/10.1016/j.ijpharm.2004.05.017
- Taib M.N., & Julkapli N.M. (2019). Dimensional stability of natural fiber-based and hybrid composites.
- Tavana H., Lam C., Grundke K., Friedel P., Kwok D., Hair M., Neumann A. (2004). Contact angle measurements with liquids consisting of bulky molecules. Journal of Colloid and Interface Science 279:493-502.
- Wirth J., Tahriri M., Khoshroo K., Rasoulianboroujeni M., Dentino A. R., Tayebi L. (2017). Surface modification of dental implants. Tayebi L., Moharamzadeh K. (Eds). Biomaterials for Oral and Dental Tissue Engineering (In.) Woodhead Publishing, pp. 85-96, doi.org/10.1016/B978-0-08-100961-1.00006-2.
©BiologyOnline.com. Content provided and moderated by BiologyOnline Editors.


