Hydrophobic
adj.
/haɪdɹəˈfəʊbɪk/
Lacking an affinity for water; insoluble in water; repelling water. Example is the hydrophobic lotus leaf repelling water.
Table of Contents
Hydrophobic Definition
The fear of mixing or reacting with water under a given set of reaction parameters is often referred to as hydrophobic. In general sciences, the ability of the substance to repel water is called hydrophobicity.
What does hydrophobic mean? The word hydrophobicity is derived from two Greek words “hydro”, meaning “water”, and “phobos”, meaning “fear”. In biology and chemistry, the term “hydrophobic” describes the substances that frequently exhibit the property of hydrophobicity are known as hydrophobic substances.
What makes a molecule hydrophobic? These types of molecules are nonpolar. And so if you are asked, “Are nonpolar molecules hydrophobic or hydrophilic?” Precisely, the non-polar molecules don’t tend to have separate charges, hence, no positive and negative poles are formed. Moreover, it can be concluded that the electrical charges in the non-polar molecules are equally distributed across the complete molecule. It is very well demonstrated by the scientists that “like dissolves like”. Hence, hydrophobic substances are miscible in non-polar liquids that are mainly organic solvents. Is water hydrophobic? It is worth mentioning here that, water is polar, hence the affiliation between water and hydrophobic molecules is very minute. Apart from hydrophobic materials, several superhydrophobic materials are mentioned in the literature [1].
Super-hydrophobic materials usually have a contact angle of greater than 150 degrees with water and thus they resist wetting (the ability of a liquid to maintain contact with the solid surface). However, the superhydrophobicity of molecules is not referred to as the chemical property of the matter rather is the result of the interfacial tension. The shape that is formed by the droplets of water on the hydrophobic materials is termed the Lotus effect. The most common examples of the lotus effect can be easily seen as the appearance of water droplets on the surface of lotus leaves and this is also used in textile engineering for self-cleaning purposes [2].
Hydrophobic means lacking an affinity for water; insoluble in water; repelling water. Examples of hydrophobic molecules include alkanes, oils, fats, and greasy substances in general. Compare: hydrophilic
Hydrophobic Molecules Examples
Various hydrophobic substances can be found in both the domestic and industrial sectors. Alkanes, oils, fats, greasy compounds, and the majority of organic compounds are hydrophobic in nature. The applications of hydrophobic substances include the removal of oil from aqueous solutions, oil spills managements, and the chemical separation process to separate the non-polar elements from the polar ones. It is a very common observation that when the oil or fats are mixed with water, two distinct layers are formed that will be immiscible with each other due to the fact the water is polar and fats and geese are non-polar particularly hydrophobic.
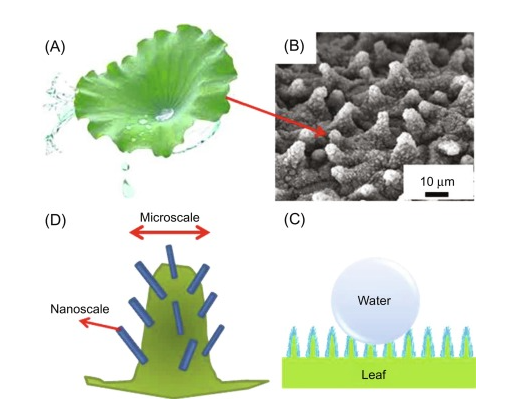
Examples of hydrophobicity can be found in both animals and plants. Many plants are hydrophobic in nature, which illustrates that there are hydrophobic coatings on the surface of the leaves. The prime responsibility of the coating is to avoid the adsorption of water and rain in the leaves, which mostly interrupt the flow of nutrients. In plants, the flow of nitrites is based on the flow of water from roots to leaves. Hence, if the surfaces of the leaves are not hydrophobic then the process of osmosis and thus osmotic pressure will be disturbed that will hugely affect the nourishment of plants. The phenomenon of hydrophobicity over the lotus leaf has been demonstrated in Figure 1. Furthermore, the SEM image of micro papillae on the lotus leaf has also been shown in the same figure as parts (b) and (c).
In birds, the process of hydrophobicity is equally vital. The hydrophobic nature of the bodies and feathers of birds avoid the penetration of water in their bodies thus avoiding excessive weight gains and assisting them in smooth flying.
Hydrophobic and Hydrophilic Substances
What is hydrophilic? Hydrophilic substances are water-loving molecules that are polar in nature. They are easily soluble in water and examples of such substances are sugar, salt, starch, and cellulose. The extent to which the surface of hydrophilic molecules attracts water molecules is called hydrophilicity.
On the other side, hydrophobic as explained earlier is water repellent and thus due to their non-polar nature, is not miscible in water. -CH3, -CH2-CH3, -R-C6H5, and C2H2 are some of the most common chemical groups found in hydrophobic substances while -OH, -COO- and -NH- are some chemical species found in hydrophilic substances.
Hydrophobic and Lipophilic
It is often seen that the terms such as hydrophobic and lipophilic come together but the two words demonstrate very different concepts. Hydrophobic substances are water repellent substances while lipophilic are fat-loving molecules. It can be seen in various literature that most of the hydrophobic substances are lipophilic in nature except silicones and fluorocarbons.
Hydrophobic Interactions
The relations between water and hydrophobes are well described under the umbrella of hydrophobic interactions. The relative mixing of water with fat is a very handy example of such interactions. The thermodynamics of hydrophobic interactions states that when a hydrophobe is dropped in an aqueous medium, the hydrogen bonds in the water molecule break to make space for the hydrophobe, but this doesn’t mean that the water molecule will react with the hydrophobic materials. Moreover, heat has to be given to the system to break the tight hydrogen bond, and thus the reaction is endothermic. New hydrogen bonds form that shape into an ice-like cage structure known as a clathrate cage around the surface of the hydrophobe. This orientation of the clathrate cage makes the system more structured and the total entropy (a measure of disorderliness) of the system is decreased. Furthermore, the strength of the hydrophobic interactions depends on the temperature, the number of carbon atoms present in the hydrophobe, and the shape and dimensions of the hydrophobic molecule [3].
Biological Importance of Hydrophobic interactions
The hydrophobic interactions are very important in protein folding making it stable and biologically active. The interactions will give a chance to protein to reduce its surface and to avoid the undesired interactions with the water molecule. Similarly, the phospholipid bilayer membranes present in every cell in the human body also depend on the hydrophobic interactions for their survival and optimum functioning.
Advantages of Hydrophobes
There are many advantages to employing hydrophobic substances for domestic and industrial applications. Hydrophobes are usually low-energy surface materials that resist wetting and have improved corrosion resistance. Such substances are used for improved moisture detection instrumentations and to prevent moisture contamination in heat trace tubing and analytical sample transfer systems. Moreover, hydrophobes are also employed in HPLC medical diagnostics improved separation and corrosion resistance systems. Similarly, the hydrophobic surfaces are used in anti-biofouling paints for boots, refining of metals, stain-resistant textiles, the separation of oil and water, in the textile industry, and the manufacture of fire retardant and waterproof clothes [4].
Measurement of Hydrophobicity
The hydrophobicity can be measured by various analytical techniques such as hydrophobic interaction chromatography, contact angle measurement, and rose bengal measurement. It is worth mentioning here that the identification of groups present in the particle is very important while measuring hydrophobicity. The most frequent method that has been used to calculate the hydrophobicity of the surface is via the calculation of contact angle in between the droplets of water and the surface itself. A contact angle of more than 90 degrees is usually maintained by the water droplet flowing over a hydrophobic surface and retains a spherical shape. Moreover, the superhydrophobic materials possess a relatively larger contact angle of above 150 degrees.
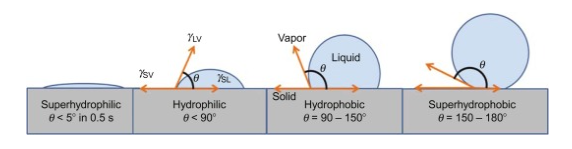
Upon contact with the hydrophilic surfaces, the droplets of water spread out far and the contact angle is generally small and is less than 90 degrees. The water contact angle between the water droplet and the various surfaces has been given in Fig 2. For super hydrophilic, the angle is less than 5 degrees, for hydrophilic the angle is less than 90 degrees and for hydrophobic and superhydrophobic the angles are 90-150 degrees and 150-180 degrees respectively. It can be concluded that the larger the contact angle in between the water droplet and hydrophobes, the stronger is the liquid-liquid interaction rather than liquid surface interaction thus making the surface hydrophobic [5].
Conclusion
It can be concluded that hydrophobic substances are those that are not miscible in water. Hydrophobes are miscible in non-polar liquids that are mainly organic solvents. Water is a polar molecule, hence the affiliation between water and hydrophobic substances is very minute and thus they form two separate and distinct layers with each other when brought in contact. Alkanes, oils, fats, and greasy compounds are hydrophobic in nature. The process of hydrophobicity can be found in both plants and birds. In plants, the interruption of the flow of nutrients is avoided by the hydrophobic layer present on the surface of the leaves that avoid the entrance of water through them. Hence, the flow of water remains from the root to the top of the plant carrying necessary nutrients from the soil to the destination. Similarly, in birds, the hydrophobicity avoids the entrances of water in the bodies of the birds via feathers and skin and aquatic animals that ultimately avoid them to become overweight and aids their smooth flying. Furthermore, the measurement of hydrophobicity can be done by calculating the contact angle between the water droplet and the surface of the hydrophobe. A contact angle of more than 90 degrees is usually maintained by the water droplet flowing over a hydrophobic surface and retains a spherical shape. Moreover, the superhydrophobic materials possess a relatively larger contact angle of above 150 degrees.
Try to answer the quiz below to check what you have learned so far about the term “hydrophobic”.
References
1. Falde Eric J, Stefan T Yohe, Yolonda L Colson, and Mark W Grinstaff Biomaterials, Superhydrophobic materials for biomedical applications. 2016. 104: p. 87-103.
2. Zhou Chan-Juan, Dan Tian, and Ji-Huan He Thermal Science, What factors affect lotus effect? 2018. 22(4): p. 1737-1743.
3. Hydrophobic Interactions. Chemistry Libre Texts, 2020.
4. Hydrophobic vs. Hydrophilic Surfaces. Silco Tek.
5. Chieng Buong Woei, Nor Azowa Ibrahim, Noraniza Ahmad Daud, and Zainal Abidin Talib, Functionalization of Graphene Oxide via Gamma-Ray Irradiation for Hydrophobic Materials, in Synthesis, Technology and Applications of Carbon Nanomaterials. 2019, Elsevier. p. 177-203.
© Biology Online. Content provided and moderated by Biology Online Editors.