Hypertonic
adj, [ˈhaɪpɚˈtɒnɪk]
Definition: Greater tension or solute concentration compared to a reference
Table of Contents
Hypertonic Definition
Hypertonic is a term used to describe an entity being in the state of hypertonicity, where there is a greater degree of tone or tension. The term “hypertonic” can be associated with the osmotic pressure exerted on the membrane due to differences in concentration between two regions, e.g., between the cytosol (inside the cell) and the surrounding fluid (outside the cell).
Another is about anatomic tone or tension (in a muscle or an organ). Hypertonicity in a muscle, for instance, would imply the presence of a greater degree of tone or tension (compared with another muscle) when muscle length changes.
Hypertonicity in Muscle Anatomy and Physiology
“Muscle tone” pertains to the physiology of muscles wherein there is motor activation happening even while at rest. This means there is a certain degree of sustained contraction even while that muscle is not voluntarily contracted, which is normal and essential in keeping the skeletal muscles firm.
A hypertonic muscle, from the word “hyper”, meaning “over”, “greater”, or “too much“, implies that such muscle is “over-activating” while at rest. Hypertonic muscles would therefore be tight and hard to the touch due to much muscle activation.
Watch this vid to learn what muscle tone is and the difference between hypertonic and hypotonic:
Hypertonic Solution
At the cellular level, tonicity refers to the property of a solution based on the osmotic force that is exerted on the membrane as a result of the concentration gradient (a gradient that forms when there are differences in solute concentrations between two solutions).
A hypertonic solution is a type of solution that has a higher concentration of solute particles compared to another solution with which it is being compared. When a cell is placed in a hypertonic solution, water will move out of the cell by osmosis, causing the cell to shrink. The significance of hypertonic solutions in biology is that they can be used to preserve cells and tissues. This is in contrast to a hypotonic solution having a lower solute concentration than another solution, causing cells to swell.
In simpler terms, it is a solution that contains a relatively higher amount of dissolved particles, such as ions or molecules, in comparison to the solution it is being compared to. This difference in solute concentration between two solutions creates a gradient that leads to osmosis, the movement of solvent molecules (usually water) from an area of lower solute concentration to an area of higher solute concentration
Watch this vid about the hypertonic solution and its difference from hypotonic and isotonic solutions:
Biology definition:
A hypertonic is a term used to refer to having a greater degree of tone, tension, or tonicity. At the cellular level, the term “hypertonic” describes a solution with a comparatively greater solute concentration than that in another solution. It could also mean that it has greater osmotic pressure than the other. At the tissue level, a muscle that is hypertonic is characterized by having a greater degree of tone or tension while at rest.
Etymology: Ancient Greek “ὑπέρ” (hupér), meaning “over” and Ancient Greek “τονικός” (tonikós), from “τόνος” (tónos).
Compare isotonic, hypotonic
In the preceding sections, hypertonicity at the cellular level will be discussed in much more detail, discussing the hypertonic solution effects on cells (both animal and plants), and their application.
Effects of Hypertonic Solutions on Cells
Homeostasis in Humans
In a hypertonic solution, such as one with a higher concentration of solutes outside the red blood cells, water molecules tend to move out of the red blood cells due to osmosis. The semipermeable membrane of the red blood cells allows water to pass through, but the higher concentration of solutes in the extracellular fluid causes water to flow out of the cells.
As a result, the red blood cells shrink and become more compact, a process known as crenation. This phenomenon highlights the significance of maintaining a balance between the intracellular and extracellular environments to prevent detrimental effects on cell function.
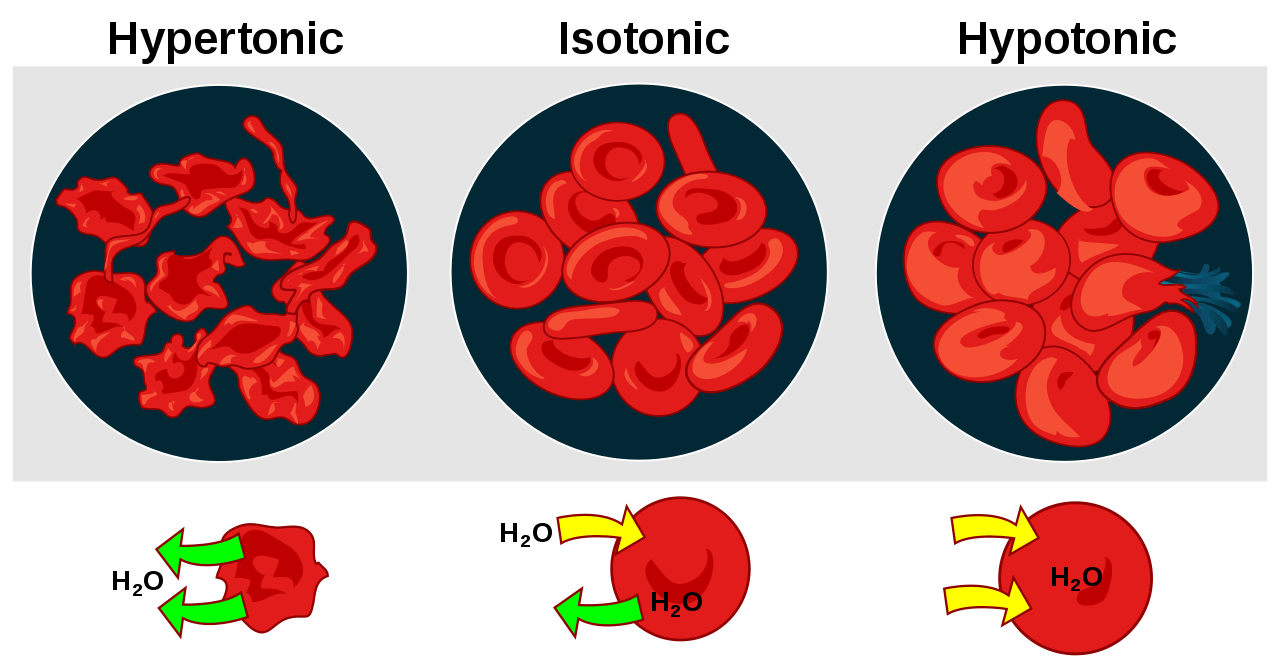
Plasmolysis in Plant Cells
In hypertonic solutions, water moves out of cells to equalize the solute concentration gradient. Plant cells experience plasmolysis, a process where the cell membrane detaches from the cell wall due to the loss of water in hypertonic environments. This leads to the collapse of the cell’s protoplast against the cell wall. Plasmolysis can have detrimental effects on the structural integrity of plant cells and may disrupt their physiological functions.
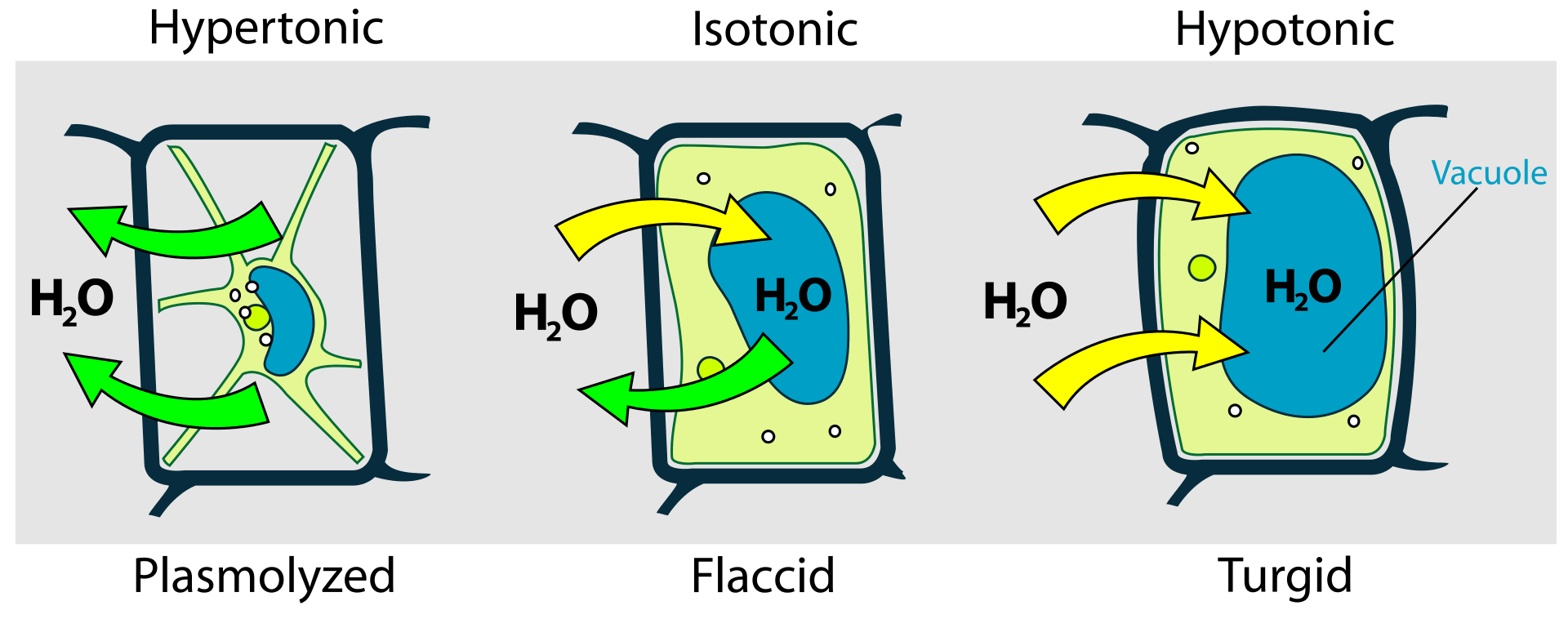
Impact on Cellular Functions
Cellular functions that rely on optimal water content, such as enzymatic reactions and organelle activities, can be compromised in hypertonic solutions. Dehydration can alter the conformation of proteins, affecting their functionality. Enzymatic processes that occur in aqueous environments may become less efficient in a hypertonic milieu.
Membrane Permeability Changes
Hypertonic conditions can alter the permeability of cell membranes. In some cases, cells may increase the expression of aquaporin proteins, which facilitate the movement of water across membranes. However, the overall effect may still lead to net water loss, resulting in cellular shrinkage.
Metabolic Responses
Cells exposed to hypertonic solutions may alter their metabolic pathways to cope with the stress. For instance, certain genes associated with osmotic stress response may be upregulated, leading to changes in gene expression and protein synthesis to enhance cellular survival.
Cell Viability and Long-Term Effects
Prolonged exposure to hypertonic conditions can impact cell viability and function. Cells may undergo irreversible damage due to excessive dehydration. In extreme cases, the osmotic stress can induce apoptosis (programmed cell death) as a protective response to prevent further damage.
Example Of Hypertonic Solutions
Hypertonic Drinks
Hypertonic drinks are beverages that have a higher concentration of solutes, such as electrolytes and carbohydrates, compared to bodily fluids. Unlike hypotonic drinks (which have a low solute concentration) and isotonic drinks (which have a similar concentration), hypertonic drinks have a higher osmotic pressure. As a result, these drinks are not typically used for immediate rehydration purposes like hypotonic or isotonic drinks.
The Isotonic sports drinks work by replacing fluids and electrolytes lost through sweating, helping to maintain hydration and performance. Hypotonic sports drinks work by replacing fluids lost through sweating. They have a lower concentration of solutes (sugar and salt) than blood, so water moves from the drink into the bloodstream by osmosis. This helps to rehydrate the body quickly and efficiently.
Characteristics and uses of hypertonic drinks
- Energy and Calorie Intake: Hypertonic drinks often contain higher amounts of carbohydrates, including sugars and sometimes complex carbohydrates. Hypertonic sports drinks work to provide a quick source of energy and calories, making them suitable for individuals engaging in endurance sports or activities that require sustained energy output.
- Recovery: Athletes and individuals who engage in intense physical activities might use hypertonic drinks during their recovery phase. The higher concentration of nutrients can aid in replenishing glycogen stores (the body’s energy reserves) and providing nutrients needed for muscle repair and growth.
- Endurance Events: Hypertonic drinks can be used during long-duration endurance events where individuals need a significant amount of energy to sustain performance over an extended period. However, it’s important to note that these drinks might not be as effective for rapid rehydration as hypotonic sports drinks or isotonic sports drinks. The best sports drink for you depends on your individual needs and preferences. Some factors to consider when choosing a sports drink include:
» The amount of electrolytes it contains
» The amount of sugar it contains - Weight Gain: Some people, particularly those aiming to gain weight or muscle mass, might use hypertonic drinks to increase their caloric intake and nutrient absorption. These drinks can provide a convenient way to consume extra calories and nutrients.
- Caution: Hypertonic drinks are not recommended for rapid rehydration or hydration during intense exercise. Their high solute concentration can draw water out of cells and lead to further dehydration if consumed without sufficient fluids
Examples at the cellular level
- Human Kidney: The human brain features a center dedicated to osmoregulation, which contains osmoreceptor proteins. These proteins are responsible for monitoring the osmotic equilibrium of the body’s cells. When these cells encounter a hypertonic environment, it indicates reduced water content in the bloodstream. This triggers the hypothalamus in the brain to release vasopressin, a hormone that prompts the kidney to enhance the permeability of its membrane. As a consequence, the kidney undergoes increased water reabsorption, elevating the volume of fluid within the bloodstream. The infusion of water into the blood vessels achieves a state-like of isotonic solution, thus upholding cell integrity.
- Osmoregulation in Sea Turtles: Both marine and freshwater turtles encounter diverse osmotic environments. Seawater boasts a notably hypertonic composition, while freshwater carries a hypotonic nature in comparison. To facilitate the optimal functioning of marine turtles, their cell cytoplasm exhibits higher hypertonicity than the surrounding seawater. Consequently, if a freshwater turtle is exposed to seawater, its cytosol becomes significantly hypotonic about the surrounding seawater. This osmotic disparity leads to the loss of water from the freshwater turtle’s body, rendering survival in seawater challenging. To adapt effectively, marine creatures, including sea turtles, have evolved mechanisms for expelling surplus salt from their systems. The salt present in the digestive tract is directed into the bloodstream, elevating the salt concentration within the turtles’ physiological fluid. This augmented salt concentration within the blood induces a state of heightened hypertonicity, preventing excessive water loss from the sea turtles’ bodies. As the surplus salt reaches the salt gland of these marine animals, it is eventually eliminated from their systems.
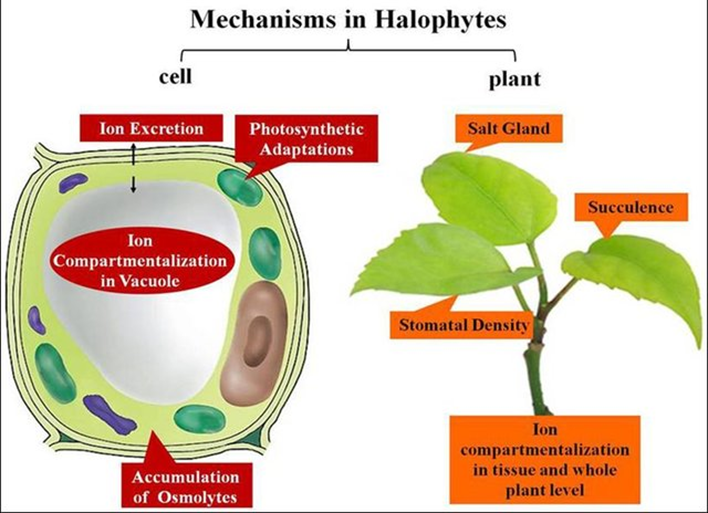
- Plants in Hypertonic Solutions: Maintaining turgidity is of utmost significance for plants to uphold their upright posture and structural integrity. Turgidity denotes the pressure exerted by water within the cell onto the cell wall. To achieve turgidity, most plants tend to favor a hypotonic setting. This preference arises because exposure to a hypotonic milieu prompts the movement of water from the external surroundings into the cells. Consequently, the cells become turgid. This phenomenon also explains why leaves droop when plants are inadequately watered. Nevertheless, there exists a category of plants that have adapted to thrive in demanding environments characterized by high salt concentrations, such as sea marshes and mangroves. These plants encounter exceedingly hypertonic conditions due to their salty surroundings. Under ordinary circumstances, a typical plant in such a hypertonic milieu would struggle to survive. However, halophytes (plants adapted to thrive in salt-rich, challenging conditions) have evolved mechanisms to flourish amid high salt concentrations. These plants can elevate the salt concentration within their root cells, resulting in the root cell cytosol becoming hypertonic. Consequently, a concentration and osmolarity gradient prompts the movement of water from the hypotonic solution towards the hypertonic solution, namely from the external environment toward the root cell. The surplus salt is either stored within the root cells or transferred to the leaves, where it can be expelled through specialized glands.
NOTE IT!
The human body is constantly trying to maintain the proper balance of water and solutes. When we sweat, we lose water and electrolytes, which can make our blood more hypertonic. To compensate, our kidneys will retain water and excrete electrolytes. This helps to keep our blood isotonic and our cells healthy.
Uses Of Hypertonic Solution
Here are the uses of hypertonic solutions in different fields:
Medical Use
- Intravenous Therapy: Hypertonic saline solutions are used in medical settings to treat conditions like severe dehydration and hyponatremia (low blood sodium levels). These solutions help restore the body fluids’ electrolyte balance and increase blood volume.
- Reducing Cerebral Edema: Hypertonic solutions can be used to reduce brain swelling in cases of traumatic brain injury or cerebral edema. By drawing excess fluid out of brain cells, these solutions can help alleviate pressure within the skull.
- Preservation of Biological Specimens: Hypertonic solutions are used in laboratories for preserving biological specimens by preventing cellular damage through osmotic balance. This is especially important in fields like histology and pathology.
Food Preservation
- Salting: The use of hypertonic solutions, often in the form of salt brines, is a traditional method for preserving food. The high concentration of salt creates an environment that inhibits the growth of bacteria and other microorganisms, thereby extending the shelf life of the food.
- Irrigation solution: Farmers can ensure optimal nutrient uptake by plants.
Industrial Processes
- Dyeing and Textile Industry: Hypertonic solutions are used in processes like dyeing and fabric treatment. They help regulate the movement of dyes and chemicals into the fabric fibers.
Biological Research
- Cell Manipulation: In biological research, hypertonic solutions can be used to manipulate the size and shape of cells for experimental purposes. This is particularly relevant in fields like cell biology and microbiology.
Agricultural Applications
- Irrigation Management: Farmers use hypertonic nutrient solutions to enhance plant growth through a technique known as fertigation. By adjusting the concentration of nutrients in the irrigation water, they create a hypertonic solution that ensures efficient nutrient uptake by plant roots.
- Saline Soil Management: In areas with saline soil, where the soil has a higher concentration of salts, plants may face difficulty in taking up water due to the hypertonic environment. Specialized crops that are salt-tolerant (halophytes) are grown in these regions, as they can adapt to such conditions.
Plant Tissue Culture
- Micropropagation: Hypertonic solutions are used in the micropropagation of plants, a technique that involves growing plant cells or tissue in a nutrient-rich medium. By controlling the osmotic balance, researchers can induce specific growth patterns and stimulate the production of multiple plantlets from a single explant.
- Stress Studies: Hypertonic solutions are employed in plant stress studies to simulate drought conditions. By exposing plants to controlled hypertonic environments, researchers can better understand the mechanisms plants use to cope with water scarcity and develop strategies to enhance drought resistance.
- Seed Germination: Some plants, especially those adapted to arid environments, require exposure to hypertonic solutions for seed germination. This process, known as stratification, involves treating seeds with a hypertonic solution to break dormancy and promote germination.
- Plant Pathology: Hypertonic solutions can be used to control certain plant diseases caused by fungi and bacteria. For instance, applying hypertonic solutions to plants affected by bacterial wilt or crown gall disease can disrupt the pathogen’s ability to spread and infect healthy tissues.
Hypertonicity in Nature
- Salt Flats: The highly saline flats found in desert environments, such as the Bonneville Salt Flats in Utah, are examples of hypertonic environments where the concentration of salt is significantly higher than that of the surrounding soil or water.
Hypertonic solutions play a critical role in various scientific, agricultural, medical, and industrial domains. Their unique properties, stemming from their higher solute concentration, have significant effects on cells and can be harnessed for a wide range of applications.
In cells and organisms, hypertonic solutions show the delicate balance between water and solute concentrations. This equilibrium is essential for cellular integrity, physiological function, and survival in various circumstances. This field helps us comprehend cellular adaptability and reaction mechanisms, enabling novel medical treatments, food preservation technologies, and life’s mysteries.
Take the Hypertonic – Biology Quiz!
References
- KhanAcademy. (2023). Osmosis and tonicity. Retrieved 17 August, 2023, from https://www.khanacademy.org/science/ap-biology/cell-structure-and-function/mechanisms-of-transport-tonicity-and-osmoregulation/a/osmosis
- Lopez, M. J., & Hall, C. A. (2020). Physiology, osmosis.
- Biology Online. (2023). Hypertonic solution. Retrieved 17 August, 2023, from https://www.biologyonline.com/dictionary/hypertonic-solution
- Veloforte. (2023). Hypertonic vs Isotonic. Retrieved 17 August, 2023, from https://veloforte.com/blogs/fuel-better/difference-between-hypotonic-isotonic-and-hypertonic-sports-drinks
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.