Hypotonic solution
n., plural: hypotonic solutions
Definition: A solution that has fewer solutes than another solution to which it is compared
Table of Contents
Hypotonic Solution Definition
What is a hypotonic solution? It refers to a solution that contains a lower amount of solute as compared with the solute concentration in the other solution across a semipermeable membrane.
Here, it is important to understand what a ‘solution’ means in science. A solution in science is referred to as a homogenous system made up of two or more constituents. The constituents that are dissolved is known as ‘solutes’ and the other constituent that dissolves the solute is known as solvent.
Hypotonicity is a relative term wherein the property of the solution is defined relative to the other solution. In biology, the majority of times, the solution of comparison is the cytosolic fluid or the fluid present inside a cell. Thus, in biology, a solution would be defined as hypotonic when it would contain a lower amount of solutes than the cytosol of a cell. The cell membrane is the semipermeable membrane. So, what happens to a cell exposed to a hypotonic environment? Will a cell shrink in a hypotonic solution? …or will it swell? A cell exposed to the hypotonic environment will have an influx of water and as a result of this, swelling of the cell ensues.
A hypotonic solution is a solution that has lower osmotic pressure than another solution to which it is compared. It may also mean a solution that contains a lower amount of solute as compared with the solute concentration in the other solution across a semipermeable membrane. In biology, a hypotonic solution is a solution wherein a cell exposed to it will eventually swell as the water molecules tend to enter the cell by passive transport. Compare: hypertonic solution, isotonic solution.
Tonicity
To have a deeper understanding of this concept, we need to understand the concept of tonicity. Basically, tonicity is a relative behavioral term wherein the amount of non-penetrating solutes determines the behavior or the movement of the solvent molecules across a semipermeable membrane. It is very essential to understand that the property is governed only by the non-penetrating solutes and not the amount of the total solutes. Now with this clarity of the concept of tonicity, we can understand three different types of solutions, i.e., hypotonic, hypertonic, and isotonic.
Hypertonicity
The prefix to the word tonicity is ‘hyper’, which means “more” or “excess”. Thus, a solution containing a higher amount of the non-penetrating solutes in comparison to the other solution across a semipermeable membrane. Consider a cell placed in the hypertonic solution. In a hypertonic environment, the amount of non-penetrating solutes in the solution outside the cytosol would be higher than the cytosolic concentration. As a result of this concentration difference, an osmotic gradient is generated across the semipermeable membrane. This results in the efflux (“flowing out”) of the solvent leading to shrinkage of the cell.
Isotonicity
Here, the prefix to the word tonicity is ‘iso’, which means “the same”. Hence, when the two solutions across a semipermeable membrane contain the same amount of non-penetrating solutes, they are known to be isotonic. In such a scenario, due to the absence of an osmotic gradient, a cell will neither shrink nor swell since there would be no net movement of the solvent molecules. With respect to blood serum, any solution having tonicity in the range of 280 – 300 mOsm/liter is referred to as isotonic with blood. 0.9% sodium chloride is the classical example of an isotonic solution.
Hypotonicity
The prefix to the tonicity (which means push or thrust) is ‘hypo’, which means “low”. In biology, hypotonic is defined as solutions having a low amount or concentration of the non-penetrating solutes in comparison to the other solution across a semipermeable membrane. So, if a cell is placed in a hypotonic solution wherein the amount of non-penetrating solutes in the solution are less and water concentration is higher than that present in the cytosol. As a result of the hypotonic environment, an osmotic gradient is generated that results in the movement of the solvent or water into the cell resulting in the swelling of the cell. Thus, a cell placed in a hypotonic solution will swell and eventually lyse. But what does lysis mean?
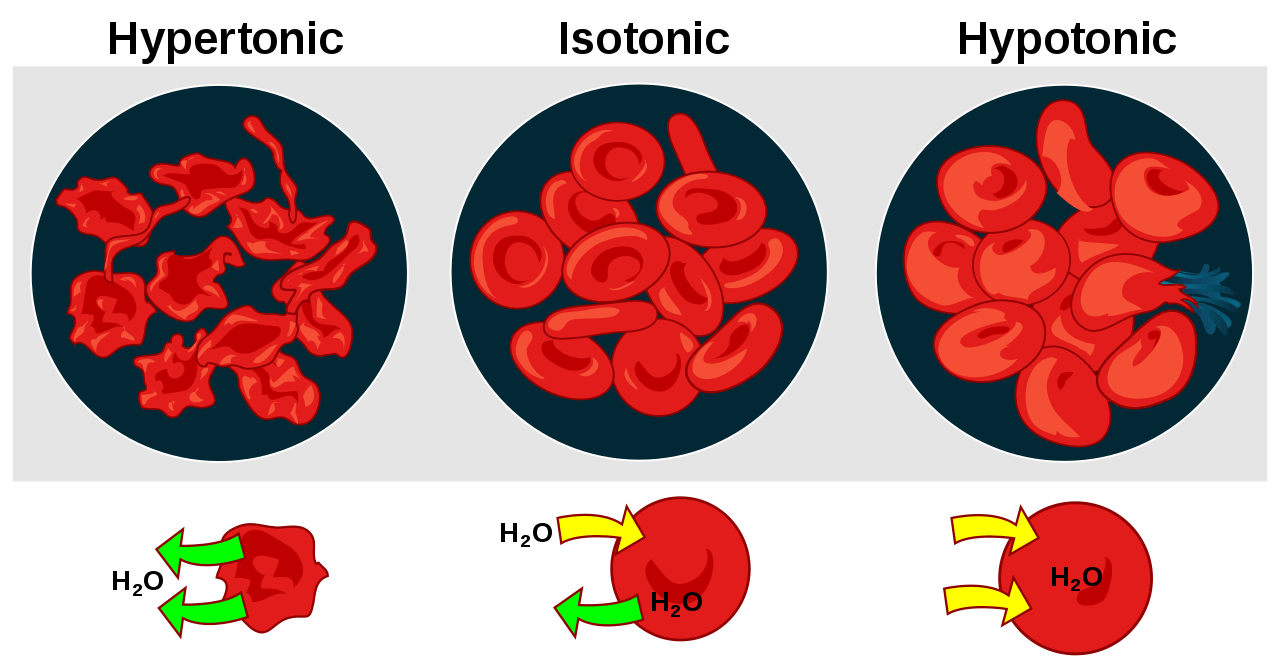
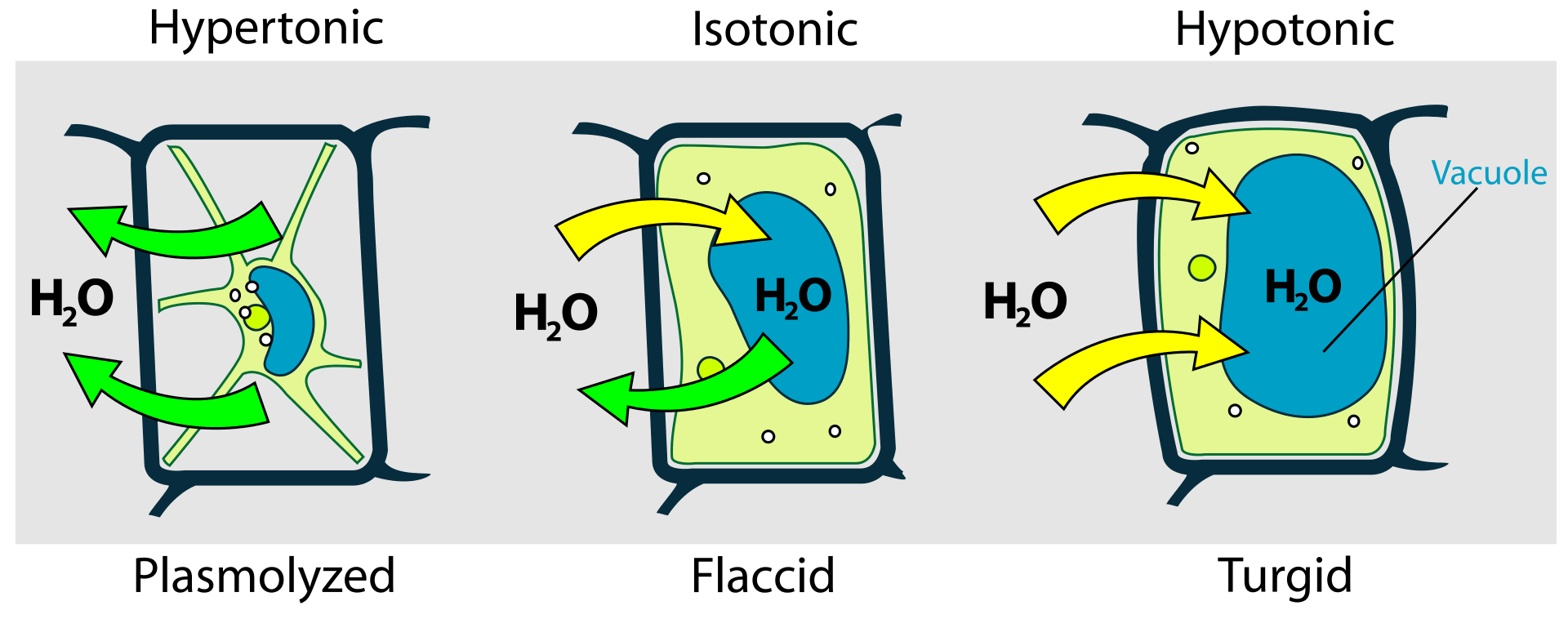
Figure 1: Difference in the behavior of animal cell and plant cell in hypotonic, isotonic, and hypertonic conditions.
‘Lysis’ is defined as the disruption of the cellular membrane which could be induced by osmotic gradient, viral, or enzymes. Lysis is the process of the activity to lyse. ‘Lyse’ is defined as the disintegration of large particles into smaller ones. Thus, these agents are described as lytic which can result in complete disruption of the cellular membrane rendering cell death.
Herein, it is important to have clarity between similar terms: plasmolysis and cytolysis. Plasmolysis is the shrinkage of the cell when placed in a hypertonic environment due to the efflux of the water from the cells. In animal cells, due to the absence of any cell wall, the cell shrinks.
However, in the case of plant cells, water efflux from cells results in the rupture of the cellular membrane from the cell wall and the creation of gaps or pockets between the cell wall and the cellular membrane. Cytolysis is observed when a cell is placed in a hypotonic solution. Due to the osmotic gradient, the water molecules will move into the cell resulting in swelling of the cell which will eventually burst and lyse. Cytolysis is observed only in animal cells and protozoa.
Depending on the cell involved the cell lysis can be termed as “haemolysis” (lysis of the red blood cells), “oncolysis” (lysis of cancerous cells), etc.
The presence of a cell wall and vacuoles protects the plant cell from cytolysis. The vacuoles take up the excess water, pushing the cellular membrane against the cell wall, which in turn exert counter thrust known as turgor pressure. Thus, plasmolysis and cytolysis are two opposite conditions that occur in hypertonic and hypotonic conditions respectively.
Watch this video below to understand the figurative representation of the fate of a cell in hypertonic vs hypotonic solutions.
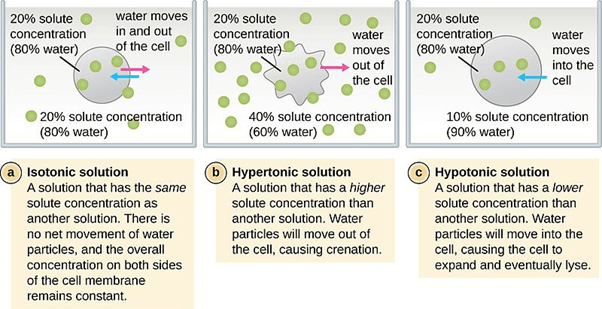
Hypotonic Solution Examples
In biology, hypotonic solutions are classified with reference to blood serum. With respect to blood serum, solutions having osmolarity less than 280 mOsm/liter are referred to as hypotonic solutions. Hypotonic saline i.e., 0.45% sodium chloride or 0.25% sodium chloride with or without dextrose, 2.5% dextrose solution, etc are some of the examples of the hypotonic solutions that are hypotonic with respect to blood serum and are used as hypotonic intravenous solutions.
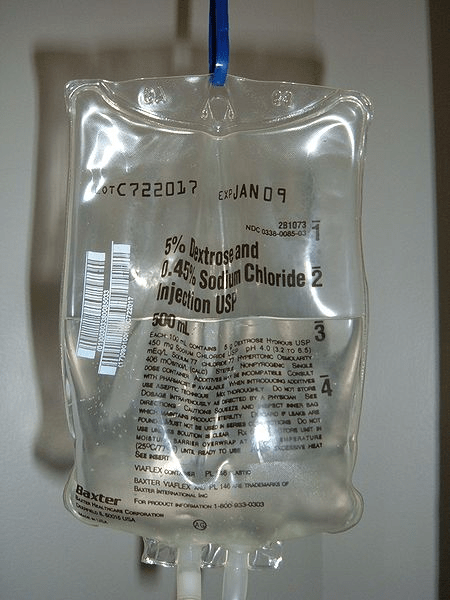
Is water a hypotonic solution?
Water is the archetypal example of a hypotonic solution. Although, again this will be subjective to the solution that is compared. Water is a solvent and purified distilled water will always be hypotonic in comparison to the aqueous solution of a solute containing any amount of the solute. Purified distilled water is devoid of any substance and hence it is considered to be hypotonic to any aqueous solution of a solute.
Biological Importance of Hypotonic Solutions
Tonicity is essential for maintaining life processes. Protists, like paramecia and amoebae, are able to retain the rigid structure due to tonicity regulation, even though they do not possess a cytoskeleton or a cell wall. These protists generally live in the hypotonic environment, and thus continuous water influx occurs. To maintain the cell structure and prevent the cell lysis, these protists have a specialized organ known as contractile vacuoles that function to accumulate an excess of water from the cell and eventually throw this excess water.
In a hypotonic environment continuous influx of water results in the generation of turgor pressure in plant cells. The plants utilize this turgor pressure to provide structure and rigidity to their structure.
Fungi (like mushrooms) and plants regulate their surroundings in order to maintain hypotonic conditions in their cell. As a result of the hypotonic environment, the influx of water will result in the generation of turgor pressure. Thus, cells remain in erect condition, maintaining the rigidity of their structure.
The plants also utilize this pressure for transporting water throughout their plant body i.e. from roots to the top stem of the plants. Thus, when plants are not watered for a long time, a hypertonic environment is created around them and they lose the turgor pressure giving a wilted appearance.

On re-watering such plants, turgor pressure is regenerated and plants regain their shape and structure. Marshy areas and mangroves have a highly hypertonic environment due to high salt content. A normal plant will wilt away in such extreme hypertonic conditions. However, in plants that exist in marshy areas, mangroves have adapted themselves to create a hypertonic cytosolic condition in their root cells. Thus, a resultant hypotonic external environment around roots helps these plants to absorb water from the surroundings.
Read: Plant Water Regulation (Tutorial)
Similarly, all aquatic animals living in seawater or freshwater are equipped to control the effect of osmosis through a mechanism known as osmoregulation. Due to this osmoregulation, salt content in the water is critical for the aquatic life in any water body. Sea turtles have adapted themselves to create a hypertonic internal environment with the help of salt glands. As a result of the hypertonic internal environment, the external environment relatively becomes hypotonic to them and thus helping these marine animals to survive even in the highly hypertonic environment.
Read: Animal Water Regulation (Tutorial)
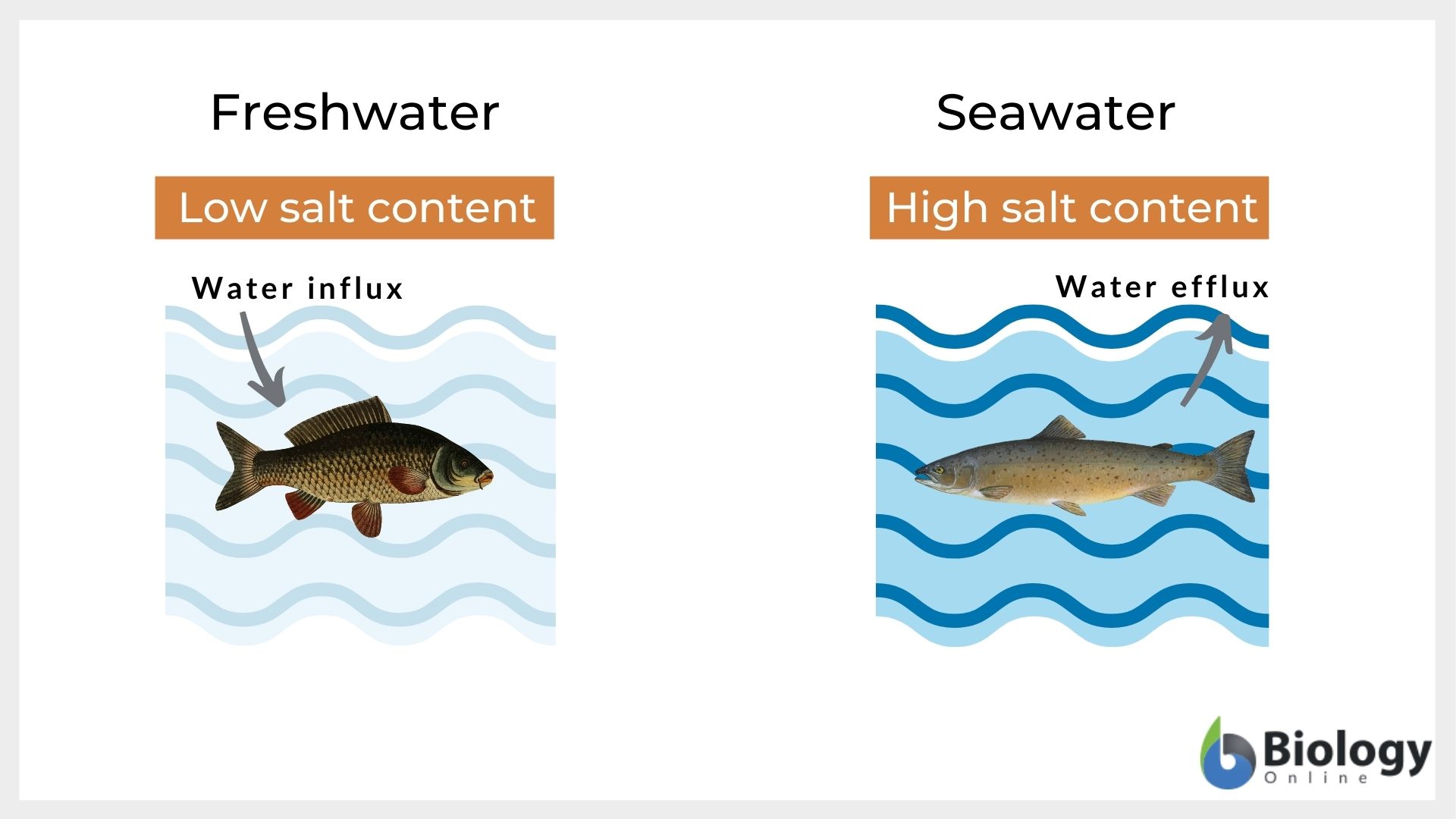
Tonicity and the osmotic gradient is the reason why freshwater fishes cannot survive in the seawater and vice versa. Freshwater is one of the examples of a hypotonic solution. Thus, the cells of the freshwater fishes have a higher salt concentration than their surrounding river or lake water. These fishes have adapted and developed a system to continuously flush out the excess water from their body. However, if freshwater fishes are exposed to seawater, freshwater fishes would have hypotonic cells in comparison to the external hypertonic environment. In such conditions, the removal of water from their body will occur and that would eventually dehydrate them and perish. Thus, fluctuation in the salt content of water drastically affects the fish population in any water body.
Medically, loss of the excess amount of sodium in comparison to the loss of water results in reduced serum osmolarity thereby leading to a condition known as hypotonic dehydration (or hyponatremia). Reduced serum osmolarity results in the influx of water from extracellular space to intracellular space thereby leading to cellular swelling and edema. This sodium imbalance results in the manifestation of neurological symptoms like nausea, headache, confusion, unconsciousness, weakness or disappearance of tendon reflex, stupor, and lethargy eventually leading to coma, and death. It is important to understand that hypotonic dehydration is actually cellular swelling and edema due to excessive water retention. This condition can occur due to excessive loss of fluid due to wound or burns, chronic diarrhea, Addison’s disease, renal tubular acidosis, chronic use of intravenous hypotonic fluids or regular saline in patients, Cystic fibrosis, and chronic use of diuretics. As commonly prescribed by the physicians, the treatment of hypotonic dehydration is initiated with 3% hypertonic saline or 0.9% isotonic saline (depending on the chronicity of the condition) along with continuous monitoring of serum sodium levels in order to prevent myelinolysis.
Try to answer the quiz below to check what you have learned so far about hypotonic solution.
References
- Lang, I., Sassmann, S., Schmidt, B., & Komis, G. (2014). Plasmolysis: Loss of Turgor and Beyond. Plants (Basel, Switzerland), 3(4), 583–593. https://doi.org/10.3390/plants3040583
- McNab, S., Ware, R. S., Neville, K. A., Choong, K., Coulthard, M. G., Duke, T., Davidson, A., & Dorofaeff, T. (2014). Isotonic versus hypotonic solutions for maintenance intravenous fluid administration in children. The Cochrane database of systematic reviews, (12), CD009457. https://doi.org/10.1002/14651858.CD009457.pub2
- Caldwell, F. T., & Bowser, B. H. (1979). Critical evaluation of hypertonic and hypotonic solutions to resuscitate severely burned children: a prospective study. Annals of surgery, 189(5), 546–552. https://doi.org/10.1097/00000658-197905000-00002
- Lodish H, Berk A, Zipursky SL, et al. (2000). Molecular Cell Biology. 4th edition. New York: W. H. Freeman; Section 15.8, Osmosis, Water Channels, and the Regulation of Cell Volume. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21739/
- Malińska, L., Rybska, E., Sobieszczuk-Nowicka, E., & Adamiec, M. (2016). Teaching about Water Relations in Plant Cells: An Uneasy Struggle. CBE life sciences education, 15(4), ar78. https://doi.org/10.1187/cbe.15-05-0113
©BiologyOnline.com. Content provided and moderated by BiologyOnline Editors.