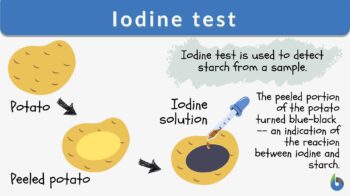
Iodine test
n., [ˈaɪ.əˌdaɪn test]
Definition: A test for the presence of starch
Table of Contents
Iodine Test Definition
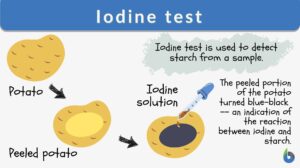
The iodine test is a chemical reaction-based identification test for starch. In this test, iodine and starch form a distinct blue-black colored complex.
What does iodine test for?
This test helps to identify the presence of starch in a sample. It also helps to distinguish between mono– or disaccharides from polysaccharides (glycogen, dextrin, and amylase).
Objectives of Iodine Test
Identification of the presence of starch in the sample provided, using the Iodine test.
Biology definition:
Iodine test is a test for detecting the presence of starch. The sample turns blue-black in color when a few drops of potassium iodide solution are placed on the sample. The reaction is due to the formation of polyiodide chains from the reaction of starch and iodine. The amylose in starch forms helices where iodine molecules assemble, forming a dark blue or black color. When starch is broken down or hydrolyzed into smaller carbohydrate units, the blue-black color is not produced. Therefore, this test can also indicate the completion of hydrolysis when a color change does not occur. Synonym: Starch-iodine test. See also: hydrolysis
Principle of Iodine Test
Before understanding the principle behind the iodine test, let us briefly understand starch and its chemistry.
Starch is a polysaccharide. Glucose synthesized by plants is stored as starch. Starch is found in abundance in potatoes and cereals (oats, barley, rice, wheat).
Natural starch contains two monomeric units: Amylose (10-20%) and Amylopectin (80-90%). Chemically both monomers are made up of D-glucose subunits. However, the positioning of the D-glucose unit in Amylose and Amylopectin is different.
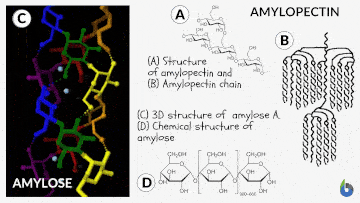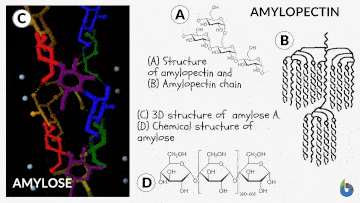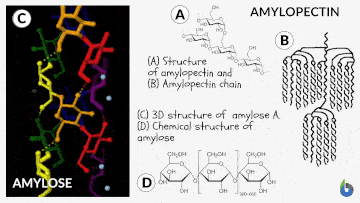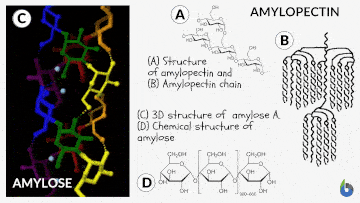
Table 1: Difference between Amylose & Amylopectin | |
|---|---|
| Amylose | Amylopectin |
| Straight chain polymer made up of D-glucose subunits | Branched-chain polymer made up of D-glucose subunits |
| In Amylose, glucose units are linked by α-1,4 glycosidic linkages | In Amylopectin, glucose units are connected by α-1,6 glycosidic linkages |
| 20% of starch is amylose | 80% of starch is amylopectin |
| Amylose is the water-insoluble fraction of starch | Amylopectin is the water-soluble fraction of starch |
| Does not exhibit gelling on the addition of hot water | Exhibit gelling on the addition of hot water |
| It gives blue color with iodine solution | It does not give blue color with iodine solution |
The chemistry behind the Iodine test
The basic principle involved in the iodine test is that Amylose interacts with starch to form a blue-black colored complex with the iodine.
The helical structure of Amylose forms a charge transfer (CT) complex with iodine, wherein iodine is present inside the spiral or helical structure of the Amylose. Therefore, for this test, iodine in water, i.e., an aqueous solution of molecular iodine (I) and potassium iodide (KI), which is known as Lugol’s iodine, is used. Interestingly, this is also known as the IKI solution.
I + KI = IKI solution
Now, let us understand the iodine purpose in Lugol’s iodine test.
Molecular iodine or iodine molecule, i.e., I2 molecule, is insoluble in water. Therefore, potassium iodide is used for making the laboratory reagent.
Potassium iodide dissociates, to form iodide ions. Iodide ions combine to form triiodide ion (I3–), which further associate to create polyiodide ions (In–) in solution. In jist, I3–chemistry is responsible for the generation of In– ions.
Polyiodide ions are negatively charged and can be triiodide (I3–), pentaiodide (I5–), or Heptaiodide (I7–). These polyiodide ions act as charge donors and form a complex with Amylose. The benchtop Lugol’s iodine solution is brown in color. However, in the charge transfer complex of polyiodide ions and Amylose, electrons absorb light energy and get excited to a higher energy level. The complementary color to the light energy absorbed by the charge transfer complex is perceived as a blue-black color by the human eye.
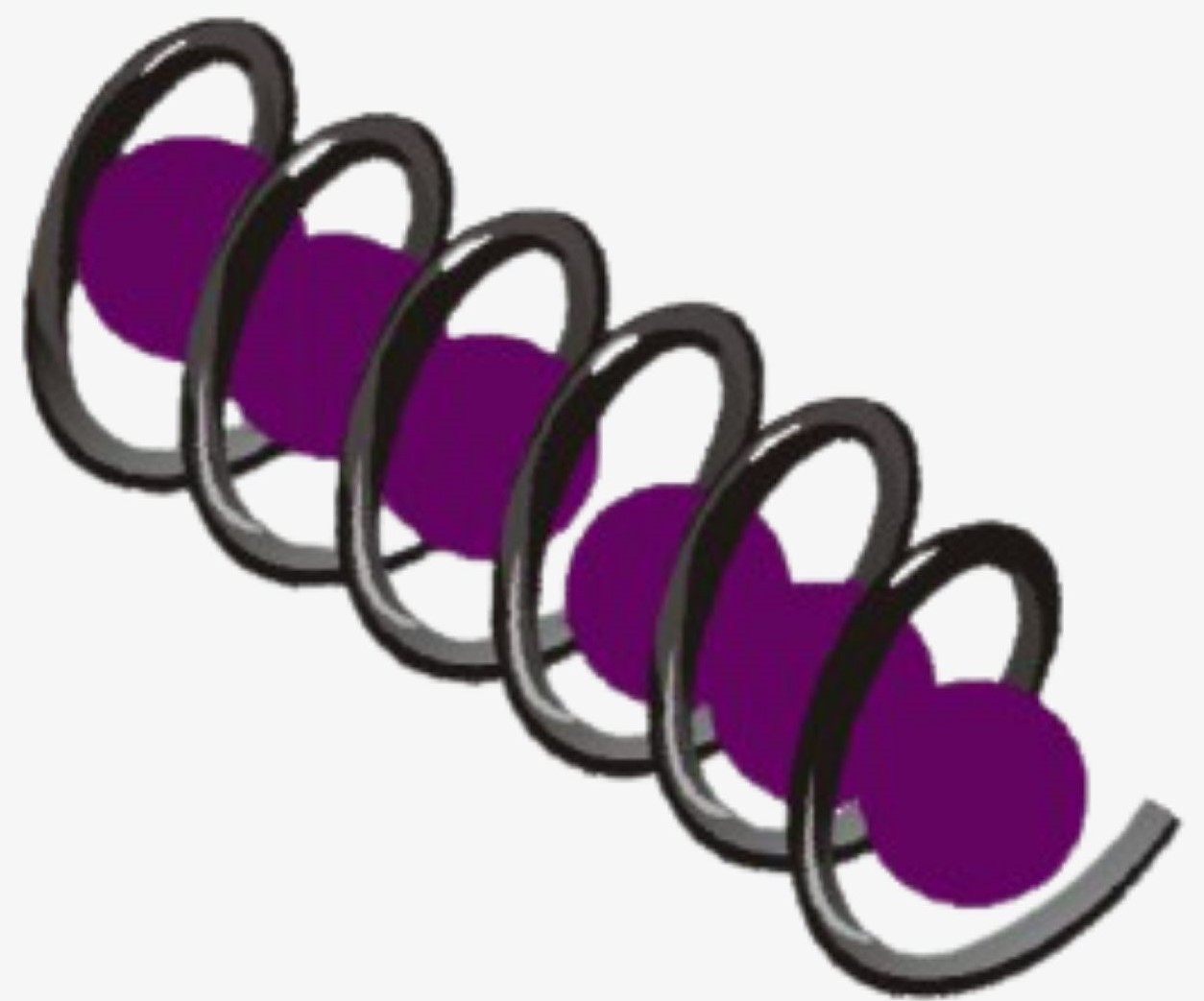
So, the benchtop iodine color is brown in color. The polyiodides (I3–, I5–, I7–) are colorless, and the amylose-iodide complex is blue-black.
The Iodine starch reaction is characteristic, and hence it is used to identify starch in a sample. Thus, the presence of starch can be confirmed using an IKI indicator.
The principle of the iodine starch test also forms the basis of all the iodometric titrations wherein the starch indicator is used.
By increasing the temperature and in the presence of water-miscible solvents like isopropyl alcohol, ethanol, etc., the blue color’s intensity decreases. This is because an increase in temperature results in the dissociation of the amylose-iodine complex. However, as the temperature falls, i.e., on cooling, the helical structure of the amylose-iodine complex is reformed, resulting in the regeneration of the blue-black color complex (Figure 2).
Requirements for Iodine test for starch
For carrying out the test for identification of starch, you would need
- Test sample
- Iodine solution or Lugol’s reagent*
- Test tubes
- Test tube stand
- Water bath
- Vortex mixer
- Dropper
Procedure of Iodine Test
The steps of the Iodine Test:
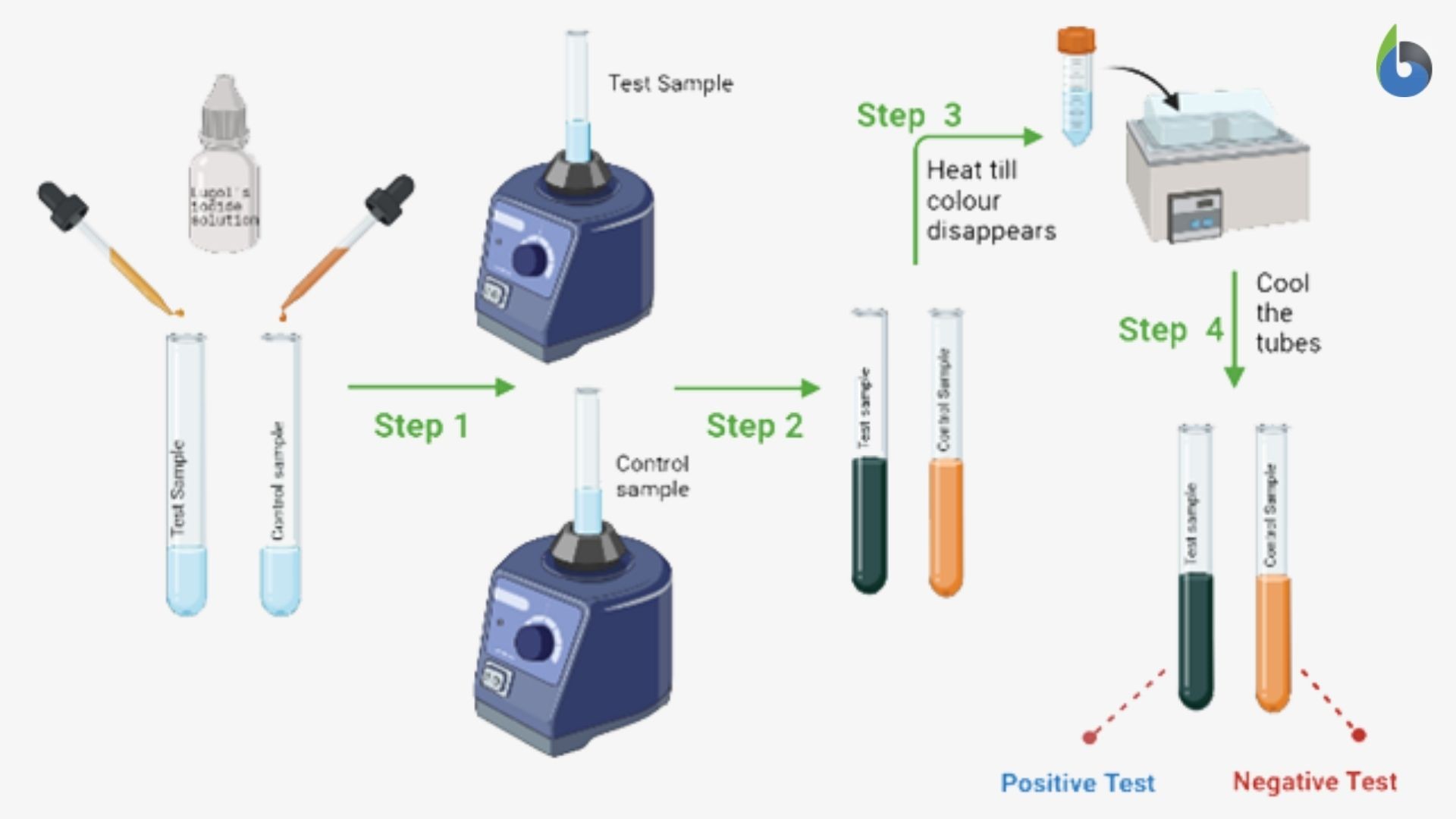
- Take two test tubes and label your test tubes as- test sample and control sample
- Take a small sample (solid sample:500 mg -1000mg; liquid: 1ml) in a clean and dried test tube labeled as a test sample.
- Take 1ml of the purified water in the clean and dried test tube labeled as the control sample.
- Add 2-3 drops of Lugol’s iodine solution to both the test tubes and mix it thoroughly on a vortex mixer.
- Observe the color that develops in both the test tubes.
- The test tubes should then be heated on a water bath until the color disappears.
- Allow the test tubes to cool down completely and observe the color in both the test tubes
Observation
Observe and note color changes in the test tubes. One may observe the following colors in the sample:
- Blue-black color
- Reddish-brown color
Watch the vid below to see how it is used to detect starch from different food samples.
Result and Interpretation of Iodine Test
Based on the observation in the color change of the samples, the following may be concluded as the iodine test for starch results:
- The appearance of blue-black color indicates the presence of the starch in the sample, i.e., a positive iodine test (Figure 3).
- The brown color or no change in the color of the sample indicates the absence of the starch in the given sample, i.e., a negative iodine test (Figure 3).
Uses of Iodine Test
What is the purpose of the iodine test? Why is it an essential laboratory procedure?
- An iodine test can be used for the detection of starch in a given sample.
- The iodine test can help to distinguish starch from monosaccharides, disaccharides, and other polysaccharides.
- The iodine test is used for distinguishing between starch, glycogen, and carbohydrates.
- Blood iodine testing is used for detecting hyper– or hypothyroidism in a patient.
- The principle of iodine test for starch is also used in starch hydrolysis test.
- The iodine test forms the basis of iodometric titrations wherein a starch indicator is used.
Limitations of Iodine Test
- One of the major limitations of the iodine test is that the test is qualitative. That means one can detect the presence or absence of the starch in the sample. However, the amount of starch present in the sample can not be estimated using the iodine test.
- The other limitation is that under acidic conditions, the starch hydrolysis. Thus, the iodine test will not be valid for acidic samples.
- The iodine test cannot be performed on a very dark-colored sample, as the color changes will not be detectable in such samples.
Important Points to Remember
- Lugol’s iodine solution is sensitive to light and should be stored in a dark amber-colored bottle in a dark place.
- The iodine test is a characteristic test for starch only. For example, the cellulose will not change color with Lugol’s iodine solution.
- The test is sensitive to changes in temperature
Try to answer the quiz below to check what you have learned so far about the iodine test.
References
- Bailey, J. M., & Whelan, W. J. (1961). Physical properties of starch. I. Relationship between iodine stain and chain length. The Journal of biological chemistry, 236, 969–973.
- Du, X., An, H., Liu, Z., Yang, H., & Wei, L. (2014). Probing starch-iodine interaction by atomic force microscopy. Scanning, 36(4), 394–400. https://doi.org/10.1002/sca.21131
- Hansen, C., Wayment, B., Klein, S., & Godfrey, B. (2018). Iodine-Starch test for assessment of hyperhidrosis in amputees, evaluation of different methods of application. Disability and rehabilitation, 40(25), 3076–3080. https://doi.org/10.1080/09638288.2017.1367965
- Mikhalev I. D. (1966). Iodnaia proba i ee klinicheskoe znachenie [The iodine test and its clinical importance]. Laboratornoe delo, 7, 387–389.
- Otto, H., & Schwerdtfeger, W. (1956). Die Jodserumprobe, eine brauchbare Schnellserumlabilitätsreaktion für die Allgemeinpraxis [Blood iodine test, a useful rapid test of blood lability in general practice]. Zeitschrift fur die gesamte innere Medizin und ihre Grenzgebiete, 1
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.