Krebs cycle
n., plural: `+ cycles
[krɛbz saɪ.kl̩]
Definition: a series of chemical reactions in cellular respiration that converts acetyl-CoA, such as into energy-rich molecules
Table of Contents
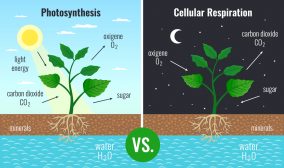
Krebs cycle, also known as the citric acid cycle or tricarboxylic acid (TCA) cycle, is a fundamental metabolic pathway that occurs in the mitochondria of eukaryotic cells and the cytoplasm of prokaryotic cells. It plays a central role in the aerobic respiration process, where cells obtain energy from glucose and other organic molecules by breaking them down and capturing the released energy in the form of ATP.
Krebs Cycle Definition
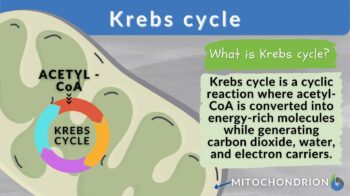
Krebs cycle is a cyclical metabolic pathway that oxidizes acetyl CoA, a two-carbon molecule derived from various fuel sources such as glucose, fatty acids, and amino acids. It extracts high-energy electrons from the acetyl CoA molecules and transfers them to electron carriers, such as nicotinamide adenine dinucleotide (NAD⁺) and flavin adenine dinucleotide (FAD), which become reduced to NADH and FADH₂, respectively. These electron carriers then participate in the subsequent oxidative phosphorylation and electron transport chain reactions, leading to the production of ATP.
Kreb cycle discovery
Albert Szent-Györgyi‘s research in the 1930s led to the establishment of several components and reactions of the citric acid cycle. His work focused on fumaric acid, a crucial element of the cycle, which earned him the Nobel Prize in Physiology or Medicine in 1937. Szent-Györgyi’s discovery was based on the study of pigeon breast muscle, known for its enduring oxidative capacity even after being broken down in the Latapie mill and released in aqueous solutions. This exceptional characteristic made pigeon breast muscle an ideal subject for investigating oxidative reactions.
In 1937, at the University of Sheffield, Hans Adolf Krebs and William Arthur Johnson finally identified the citric acid cycle, leading to Krebs being awarded the Nobel Prize for Physiology or Medicine in 1953. Consequently, the cycle is sometimes referred to as the “Krebs cycle” in honor of his significant contribution.
Krebs Cycle as a part of Cellular Respiration
Cellular respiration occurs within cells and involves a catabolic reaction. This biochemical procedure breaks down nutrients to unlock energy, which is stored as ATP, while simultaneously releasing waste products. Oxygen is necessary for aerobic respiration. In aerobic respiration, oxygen is required. In the process, glucose is oxidized to carbon dioxide, and oxygen is reduced to water. The energy released in the process is stored in the form of ATPs. 36 to 38 ATPs are formed from each glucose molecule (Budin et al., 2018).
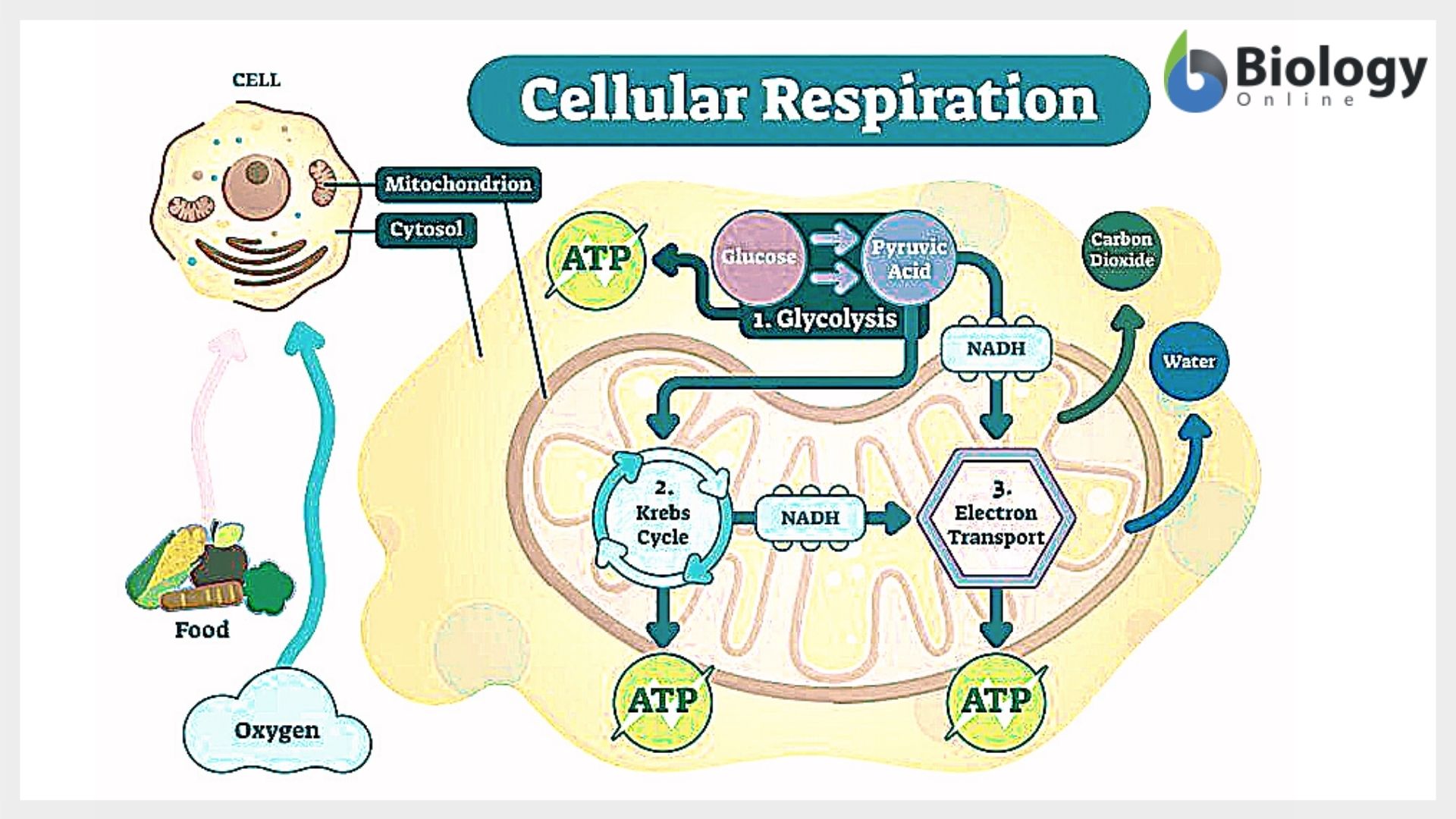
The process of cellular respiration encompasses four distinct stages. The four stages are:
- Glycolysis: When a glucose molecule goes through a special process called partial oxidation, it turns into two pyruvate molecules. This whole thing happens inside the cytosol of the cell.
- Formation of Acetyl CoA: After glycolysis, the pyruvate molecules move into the mitochondria’s inner part called the mitochondrial matrix. In there, they go through something called oxidative decarboxylation and become two molecules of Acetyl CoA. This change is made possible by a special enzyme called pyruvate dehydrogenase.
- Krebs Cycle: The Krebs cycle is like a superhighway where carbohydrates, proteins, and lipids get fully oxidized. They turn into acetyl coenzyme A or other molecules in the cycle. The Acetyl CoA molecules produced during this process enter the cycle. Glucose gets completely broken down here. The acetyl CoA combines with a 4-carbon compound called oxaloacetate and forms a 6-carbon molecule called citrate. Along the way, two molecules of CO2 are released, and oxaloacetate gets reused. The energy that is released gets stored in special molecules like ATP, NADH, and FADH2, which have high energy levels.
- Electron Transport System and Oxidative Phosphorylation: In this step, ATP gets generated as electrons move from molecules like NADH and FADH2 (which are produced in glycolysis, the citric acid cycle, and fatty acid oxidation) to a molecule called molecular O2. This transfer happens through a series of electron carriers. When oxygen combines with these electrons, it turns into water (H2O). This whole process takes place in the inner membrane of mitochondria.
Energy budget of cellular respiration
The maximum amount of ATP that can be produced from the oxidation of one molecule of glucose in glycolysis, the citric acid cycle, and oxidative phosphorylation is theoretically 38 ATP molecules. This calculation assumes that for every molecule of NADH, three molar equivalents of ATP are generated, and for every molecule of FADH2, two ATP molecules are produced.
In eukaryotes, glycolysis occurs in the cytoplasm and generates two molecules of NADH and four molecules of ATP. However, to transport two of the NADH molecules into the mitochondria, two ATP molecules are required. As a result, the net production of ATP due to this energy expenditure in glycolysis becomes 36 molecules.
Additionally, inefficiencies in oxidative phosphorylation occur due to proton leakage across the mitochondrial membrane and slippage of the ATP synthase/proton pump. These inefficiencies decrease the ATP yield from NADH and FADH2, resulting in approximately 2.5 ATP per NADH and 1.5 ATP per FADH2. Consequently, the total net production of ATP is further reduced to around 30 molecules.
With a revised assessment of the proton-to-ATP ratios, the estimated ATP yield per glucose molecule is approximately 29.85 ATP.
Watch this vid about Krebs cycle:
Biology definition:
Krebs cycle is a cycle of reactions catalyzed by enzymes in which the pyruvate derived from nutrients and converted to Acetyl Coenzyme A is completely oxidized and broken down into carbon dioxide and water to produce high-energy phosphate compounds, which are the source of cellular energy. In eukaryotes, the Krebs Cycle occurs in the matrix of the mitochondrion whereas in prokaryotes, it occurs in the cytoplasm.
Quick Overview:
Cellular respiration is a series of metabolic processes wherein the biochemical energy is harvested from an organic substance (e.g. glucose) and stored in energy carriers (e.g. ATP) for use in energy-requiring activities of the cell. The major steps or processes of cellular respiration are (1) glycolysis, (2) Krebs cycle, and (3) oxidative phosphorylation. Krebs cycle is a stage of cellular respiration following glycolysis and is characterized by its decarboxylation of pyruvate.
The other names for the Krebs cycle are the citric acid cycle (because citric acid is both the first product and the final reactant) and the tricarboxylic acid cycle (because of the involvement of the three carboxyl groups).
Word origin: named after Hans Adolf Krebs, first described in 1937.
Synonyms: citric acid cycle (CTC), tricarboxylic acid cycle (TCA cycle)
Krebs Cycle Overview
Let’s have a closer look at the Krebs cycle reactants…
Acetyl-CoA: Each cycle begins with the condensation of two carbon molecules of acetyl-CoA with four carbon oxaloacetate, forming citrate. One of the primary sources of acetyl-CoA is the breakdown of sugars by glycolysis which yield pyruvate. The acetyl group of pyruvate in turn is decarboxylated by the pyruvate dehydrogenase and the coenzyme is reduced resulting in a complex generating acetyl-CoA according to the following reaction scheme:
CH3C(=O)C(=O)O− + HSCoA + NAD+ → CH3C(=O)SCoA + NADH + CO2
Lipoic acid is an essential part of a multi-enzyme complex called the pyruvate dehydrogenase complex, which catalyzes the conversion of pyruvate to acetyl-CoA before entering the Krebs cycle.
Krebs Cycle Products
The Krebs cycle generates several products, including energy-rich molecules and electron carriers. One molecule of glucose produces two molecules of acetyl CoA, leading to two turns of the Krebs cycle. The cycle produces three NADH molecules, one FADH₂ molecule, one ATP molecule (through substrate-level phosphorylation), and two carbon dioxide molecules.
- NADH: During the cycle, NAD+ is reduced to NADH. This is a high-energy electron carrier that can be used in the electron transport chain (ETC) to generate ATP.
- FADH2: In addition to NADH, the cycle produces FADH2, another high-energy electron carrier for the ETC.
- ATP: 1 ATP is produced in the conversion of succinyl CoA to succinate. Directly, the Krebs cycle does not produce other ATPs. However, the high-energy electron carriers NADH and FADH2 generated during the cycle enter the electron transport chain (ETC), which is coupled to ATP synthesis via oxidative phosphorylation. The electrons from NADH and FADH2 are passed along the ETC, creating a proton gradient across the inner mitochondrial membrane, which ultimately drives the synthesis of ATP.
- Carbon dioxide (CO2): The oxidation of acetyl-CoA results in the release of two molecules of CO2 per cycle. Removal of CO2 or decarboxylation of citric acid takes place at two places:
- In the conversion of isocitrate (6C) to α-ketoglutarate (5C)
- In the conversion of α-ketoglutarate (5C) to succinyl CoA (4C)
Energy Budget of Krebs cycle
The net result of one turn of the Krebs cycle can be summarized in terms of products per acetyl-CoA molecule:
» 3 NADH molecules
» 1 FADH2 molecule
» 1 ATP molecule (via substrate-level phosphorylation)
» 2 CO2 molecules
Since two acetyl-CoA molecules are generated from one glucose molecule (through glycolysis), the net products from one glucose molecule are doubled. Keep in mind that the actual number of ATP molecules generated from the NADH and FADH2 produced in the Krebs cycle can vary depending on factors such as shuttle mechanisms (e.g., malate-aspartate shuttle or glycerol 3-phosphate shuttle) and the proton gradient established during the ETC. Each molecule of NADH can form 2-3 ATPs and each FADH2 carrying two hydrogen atoms gives 2 ATPs on oxidation in the electron transport chain. The precise ATP yield is estimated to be around 30-32 ATP molecules per glucose molecule in eukaryotic cells.
Krebs cycle equation:
![]()
Overview of Process
The Krebs cycle serves as a central hub in cellular respiration, connecting various metabolic pathways and supplying the electron transport chain with high-energy electron carriers. It takes place in the mitochondrial matrix, and is tightly integrated with oxidative phosphorylation, the final step of cellular respiration. It involves eight enzymes responsible for completely oxidizing acetate, a two-carbon molecule in the form of acetyl-CoA, into two molecules each of carbon dioxide and water. Acetyl-CoA, derived from the catabolism of sugars, fats, and proteins, enters the citric acid cycle as the two-carbon organic product.
During the cycle’s reactions, three molecules of nicotinamide adenine dinucleotide (NAD+) are converted into three equivalents of reduced NAD+ (NADH), and one equivalent of electron carrier flavin adenine dinucleotide (FAD) is transformed into one equivalent of FADH2. Additionally, one equivalent of each guanosine diphosphate (GDP) and inorganic phosphate (Pi) is converted into one equivalent of guanosine triphosphate (GTP). Subsequently, the NADH and FADH2 produced during the citric acid cycle play a crucial role in the oxidative phosphorylation pathway, generating energy-rich ATP
The citric acid cycle begins with the transfer of a two-carbon acetyl group from acetyl-CoA to the four-carbon molecule of oxaloacetate to form a six-carbon compound (citrate). The citrate then goes through a series of chemical transformations, losing two carboxyl groups as CO2. The carbons lost as CO2 originate from what was oxaloacetate, not directly from acetyl-CoA. The carbons donated by acetyl-CoA become part of the oxaloacetate carbon backbone after the first turn of the citric acid cycle. Loss of the acetyl-CoA-donated carbons as CO2 requires several turns of the citric acid cycle. However, because of the role of the citric acid cycle in anabolism, they might not be lost, since many citric acid cycle intermediates are also used as precursors for the biosynthesis of other molecules.
Where Does The Krebs Cycle Take Place?
The Krebs cycle primarily occurs in the mitochondrial matrix, a gel-like substance enclosed by the inner mitochondrial membrane. This compartmentalization allows for efficient energy production and regulation of metabolic intermediates.
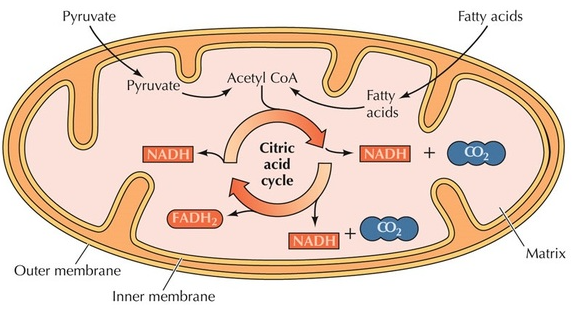
Krebs Cycle Steps
The cycle takes place in eight distinct steps, each catalyzed by a specific enzyme. Here is a detailed breakdown of the Krebs cycle (Alabduladhem & Bordoni, 2022):
- Step 1: Formation of CitrateThe cycle begins when acetyl CoA, a two-carbon molecule, combines with a four-carbon molecule called oxaloacetate. This reaction is catalyzed by the enzyme citrate synthase, which forms citrate, a six-carbon molecule.
- Step 2: Isomerization of Citrate to Isocitrate CitrateCitrate is then isomerized to isocitrate, a reaction catalyzed by the enzyme aconitase.
- Step 3: Oxidative Decarboxylation of IsocitrateIsocitrate undergoes oxidative decarboxylation, wherein a carbon dioxide molecule is released, and a reduced electron carrier, NADH, is generated. This reaction is catalyzed by the enzyme isocitrate dehydrogenase, and it transforms isocitrate into a five-carbon molecule called α-ketoglutarate.
- Step 4: Oxidative Decarboxylation of α-KetoglutarateIn this step, α-ketoglutarate is further oxidatively decarboxylated, releasing another carbon dioxide molecule and generating another molecule of NADH. The enzyme α-ketoglutarate dehydrogenase catalyzes this reaction, resulting in the formation of a four-carbon molecule called succinyl CoA.
- Step 5: Formation of SuccinateSuccinyl CoA is then converted into succinate through a substrate-level phosphorylation reaction. This means that a high-energy phosphate group is transferred from succinyl CoA to guanosine diphosphate (GDP), forming guanosine triphosphate (GTP). GTP can subsequently donate its phosphate group to adenosine diphosphate (ADP) to form ATP. The enzyme succinyl CoA synthetase catalyzes this reaction.
- Step 6: Oxidation of Succinate to FumarateIn this step, succinate is oxidized to fumarate, and an electron carrier, flavin adenine dinucleotide (FAD), is reduced to FADH₂. This reaction is catalyzed by the enzyme succinate dehydrogenase, which is embedded in the inner mitochondrial membrane.
- Step 7: Hydration of Fumarate to MalateFumarate undergoes hydration, converting it into malate. The enzyme fumarase catalyzes this reaction.
- Step 8: Oxidation of Malate to OxaloacetateMalate is oxidized, generating another molecule of NADH. The enzyme malate dehydrogenase catalyzes this final step of the Krebs cycle, converting malate back into oxaloacetate, which can then combine with another molecule of acetyl CoA to begin the cycle anew.
Regulation of Krebs Cycle
I. Allosteric regulation by metabolites
Allosteric regulation plays a significant role in the control of the Krebs cycle. In allosteric regulation, certain metabolites act as allosteric effectors, binding to specific enzymes involved in the Krebs cycle at sites other than their active sites. This binding can result in either the activation or inhibition of enzyme activity, effectively modulating the overall rate of the Krebs cycle and its metabolic output. Here are some examples of key allosteric regulators in the Krebs cycle:
- Citrate synthase: Citrate synthase is the enzyme responsible for the formation of citrate from acetyl-CoA and oxaloacetate. High levels of ATP and NADH, which indicate sufficient energy reserves, act as negative allosteric effectors, inhibiting citrate synthase and slowing down the Krebs cycle.
- Isocitrate dehydrogenase: This enzyme catalyzes the conversion of isocitrate to alpha-ketoglutarate, producing NADH in the process. High levels of ATP and NADH inhibit isocitrate dehydrogenase, while increased levels of ADP and NAD+ activate the enzyme. These allosteric regulations help maintain metabolic balance and prevent the unnecessary production of NADH when energy levels are already high.
- Alpha-ketoglutarate dehydrogenase: Alpha-ketoglutarate dehydrogenase catalyzes the conversion of alpha-ketoglutarate to succinyl-CoA, generating NADH and CO2. Like isocitrate dehydrogenase, this enzyme is also regulated by ATP, NADH, and ADP. High ATP and NADH levels inhibit the enzyme, while elevated ADP levels activate it.
- Succinyl-CoA synthetase: This enzyme converts succinyl-CoA to succinate, producing ATP or GTP in the process through substrate-level phosphorylation. Succinyl-CoA synthetase is regulated by phosphorylation and Mg2+ ions, rather than classical allosteric effectors.
- Malate dehydrogenase: Malate dehydrogenase catalyzes the conversion of malate to oxaloacetate, generating NADH. High NADH levels inhibit the enzyme, while increased NAD+ levels activate it.
Allosteric regulation allows the Krebs cycle to respond dynamically to the cell’s energy needs and metabolic state. It ensures that the cycle is efficiently regulated, preventing wasteful energy expenditure and maintaining cellular homeostasis (Gasmi et al., 2021).
II. Transcriptional regulation
The transcriptional regulation of the Krebs cycle involves the control of gene expression of the enzymes involved in this cycle. The expression of these genes is regulated by various transcription factors and signaling pathways that respond to the cell’s metabolic state and energy needs. Here’s an overview of how the Krebs cycle is transcriptionally regulated:
- Transcription factors: Transcription factors are proteins that bind to specific DNA sequences in the regulatory regions (promoters or enhancers) of target genes and influence their transcription. Several transcription factors are involved in regulating the genes encoding the enzymes of the Krebs cycle. For example, nuclear respiratory factor 1 (NRF-1) and NRF-2 are known to regulate the expression of several genes involved in oxidative phosphorylation and the Krebs cycle.
- Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α): PGC-1α is a coactivator that interacts with various transcription factors to increase the expression of genes involved in mitochondrial biogenesis and oxidative metabolism. It plays a crucial role in regulating the expression of genes related to the Krebs cycle, as well as other mitochondrial processes.
- Energy-sensing pathways: The transcriptional regulation of the Krebs cycle is closely linked to cellular energy status. Energy-sensing pathways, such as AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR), can modulate the expression of genes involved in the Krebs cycle in response to changes in cellular energy levels.
- Hypoxia-inducible factor 1 (HIF-1): HIF-1 is a transcription factor that is activated in response to low oxygen levels (hypoxia). Under hypoxic conditions, HIF-1 can upregulate the expression of genes involved in glycolysis and downregulate some components of the Krebs cycle, shifting cellular metabolism towards glycolysis for ATP production.
- Transcriptional coactivators and corepressors: Coactivators and corepressors are proteins that interact with transcription factors to enhance or inhibit their activity, respectively. They can influence the transcription of genes involved in the Krebs cycle by modulating the function of specific transcription factors.
Krebs Cycle Function
The Krebs cycle serves multiple functions in cellular respiration (Ryan et al., 2019).
- Generation of ATP: It completes the oxidation of fuel molecules, such as glucose, fatty acids, and amino acids, releasing stored energy in the form of high-energy electron carriers (NADH and FADH₂). These electron carriers then participate in oxidative phosphorylation, where ATP production occurs through the electron transport chain.
- Metabolic intermediates: Krebs cycle is an essential provider of metabolic intermediates for various biosynthetic pathways. It supplies intermediates for amino acid synthesis, fatty acid synthesis, and other cellular processes necessary for growth and maintenance.
- Interconversion of amino acids: Amino acids (metabolic products of proteins) are deaminated and get converted to pyruvate and other intermediates of the Krebs cycle. They enter the cycle and get metabolized e.g. alanine is converted to pyruvate, glutamate to α-ketoglutarate, aspartate to oxaloacetate on deamination.
- Release of carbon dioxide: As acetyl-CoA is oxidized and its carbon atoms are released, carbon dioxide is generated as a waste product.
- Regeneration of oxaloacetate: At the end of the Krebs cycle, oxaloacetate is regenerated, allowing the cycle to continue as long as acetyl-CoA and other intermediates are available.
- Interconnection with other pathways: The Krebs cycle is a central hub in cellular metabolism. It connects with various other metabolic pathways, including glycolysis, fatty acid oxidation, and amino acid metabolism.
- Reducing power for detoxification: The NADH and FADH2 generated during the Krebs cycle can act as reducing agents to facilitate detoxification processes. For example, NADH is essential for the function of various detoxifying enzymes, including those involved in the metabolism of drugs and xenobiotics.
- pH regulation: The Krebs cycle contributes to cellular pH regulation. The production of carbon dioxide during the cycle leads to the formation of carbonic acid, which can be converted to bicarbonate ions, helping to maintain the acid-base balance in the cell.
The Krebs cycle is a vital metabolic pathway in cellular respiration. It operates in the mitochondrial matrix, generating high-energy electron carriers and ATP molecules. Besides its role in energy production, the Krebs cycle also provides key intermediates for the synthesis of various cellular components. Understanding the intricacies of this cycle helps unravel the complexity of cellular metabolism and the efficient functioning of living organisms.
NOTE IT!
Did you know the Krebs cycle not only produces energy but also plays a surprising role in the production of other essential molecules?
Several intermediates of the cycle, such as alpha-ketoglutarate and oxaloacetate, are used as building blocks for the synthesis of amino acids, which are the building blocks of proteins. This demonstrates the interconnectedness of cellular processes and the versatile nature of the Krebs cycle.
Take the Krebs Cycle – Biology Quiz!
References
- Alabduladhem, T. O., & Bordoni, B. (2022). Physiology, krebs cycle. In StatPearls [Internet]. StatPearls Publishing.
- Budin, I., de Rond, T., Chen, Y., Chan, L. J. G., Petzold, C. J., & Keasling, J. D. (2018). Viscous control of cellular respiration by membrane lipid composition. Science, 362(6419), 1186-1189.
- Gasmi, A., Peana, M., Arshad, M., Butnariu, M., Menzel, A., & Bjørklund, G. (2021). Krebs cycle: activators, inhibitors and their roles in the modulation of carcinogenesis. Archives of Toxicology, 95(4), 1161-1178.
- Ryan, D. G., & O’Neill, L. A. (2020). Krebs cycle reborn in macrophage immunometabolism. Annual review of immunology, 38, 289-313.
- Ryan, D. G., Murphy, M. P., Frezza, C., Prag, H. A., Chouchani, E. T., O’Neill, L. A., & Mills, E. L. (2019). Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nature metabolism, 1(1), 16-33.
- Stubbs, R. T., Yadav, M., Krishnamurthy, R., & Springsteen, G. (2020). A plausible metal-free ancestral analogue of the Krebs cycle composed entirely of α-ketoacids. Nature chemistry, 12(11), 1016-1022.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.