Table of Contents
Definition
noun
plural: lactoses
lac·tose, ˈlæk.toʊs
A disaccharide (chemical formula: C12H22O11) made up of one galactose and one glucose, joined together via β-(1→4) glycosidic bond
Details
Discovery of lactose
Lactose has long been known and used but it was recognized as a sugar only in 1780 by the chemist Carl Scheele 1742 – 1786. Fabriccio Bartoletti 1576 – 1630 was credited as the first to be able to crudely isolate lactose in milk. Lactose accounts for the roughly 2-8% of the weight of the milk. The monosaccharide components of lactose were identified later. The first monosaccharide component identified was glucose by Heinrich Vogel 1778 – 1867 in 1812. The other monosaccharide component, galactose, was identified by Louis Pasteur 1822 –1895 in 1856.
The name “lactose” comes from the word lac or lactis (meaning “milk”) and the suffix -ose to indicate it is a sugar. Its name was given by the French chemist Jean Baptiste André Dumas in 1843. However, when Pasteur was able to isolate galactose, he referred to it as “lactose”.1 The compound was later renamed galactose (or “glucose lactique”) by the French chemist Pierre Eugène Marcellin Berthelot 1827–19072, and then he called the milk sugar as lactose, just as what we know today.
Overview
Lactose is one of the most common disaccharide carbohydrates; other examples are sucrose and maltose. Carbohydratess are a major class of biomolecules that can be classified based on the saccharide constituents. A disaccharide is a carbohydrate made up of two monosaccharides that are linked together by a glycosidic bond (glycosidic linkage).
Properties of lactose
Lactose is a white crystalline non-hygroscopic solid. Its molar mass is 342.30 g·mol−1. Its melting point is 202.8 °C. It is soluble in water. Similar to sucrose and maltose, lactose has a general formula of C12H22O11. Lactose, though, is a disaccharide made up of one galactose and one glucose. They are linked together by β-1→4 glycosidic bond, which means the covalent bond forms between the β-anomeric form of Carbon-1 (C-1) on galactose and the hydroxyl oxygen atom on C-4 on glucose.
Lactose vs. Sucrose vs. Maltose
Lactose (milk sugar), maltose (malt sugar), and sucrose (common table sugar) are the three common dietary disaccharides. As already specified earlier, the three disaccharides have the same chemical formula: C12H22O11. All three have a glucose constituent. In maltose, two glucose units make up the compound. In lactose and sucrose though, there is only one glucose unit that combines with another monosaccharide – a galactose and a fructose, respectively. In maltose, α-(1,4) glycosidic bond joins the two sugars, i.e. between C1 and C4. In lactose, β-(1,4) glycosidic bond occurs between C1 of galactose and C4 of glucose. In sucrose, α-(1,2)-β bond forms between C1 of glucose and C2 of fructose.
Both lactose and maltose are reducing sugars whereas sucrose is a non-reducing sugar. Maltose and lactose are reducing sugars because one of the monosaccharide constituents could present a free aldehyde group. As for sucrose, the glycosidic bond forms between the reducing ends of the two monosaccharide constituents. Thus, sucrose could not join any further with other saccharide units.
Dietary lactose typically comes from milk and dairy products whereas sucrose is usually from food sweetened by sugar extracted from sugar cane and sugar beet and maltose, often from the digestion of starchy food. The digestion of these sugars is aided by specific digestive enzymes, particularly lactase, sucrase and maltase. In humans, these enzymes are located on the outer surface of the epithelial cells that line the small intestine. Lactase, for instance, aids in the digestion of lactose. β -galactosidase is the counterpart of lactase in bacteria. Maltase helps digest maltose whereas sucrase helps digest sucrose. These enzymes cleave the bond between the two monosaccharide components. Of these three, sucrose is the sweetest, followed by maltose.
Common biological reactions involving lactose
Common biological reactions involving lactose
The biosynthesis of lactose involves one galactosyl unit and one glucosyl unit joined via β-1→4 glycosidic linkage. The joining of these two monosaccharides results in the release of water. Galactose is in the beta-pyranose form whereas glucose may either be alpha– or beta-pyranose.
Common biological reactions involving lactose
The process whereby complex carbohydrates are broken down into simpler forms is saccharification. It is the opposite of dehydration synthesis. In dehydration synthesis, the condensation reaction causes the glycosidic bond to form between the joining sugars to form complex carbohydrates and then water is released in the process. In saccharification, hydrolysis uses water molecule and causes the glycosidic bond to break, thereby releasing the sugar constituents.
Lactose in milk and dairy products (e.g. cheese, yoghurt, cream) are digested in the small intestine through the enzyme lactase. In bacteria, the enzyme used in the hydrolysis of lactose is β-galactosidase. Lactase is secreted by the intestinal villi. It cleaves lactose into galactose and glucose, which can then be absorbed by the enterocytes (intestinal cells), released into the bloodstream, and finally, taken up by the cells of various tissues, e.g. liver, kidney, muscle, etc.
Common biological reactions involving lactose
In humans and other mammals, mothers feed their infants with breast milk secreted by the mammary glands. Breast milk is a body fluid produced by the mammary glands of human breasts. Lactose is the primary carbohydrate in human milk. Lactose composition of human milk is about 7g/100mL.3
Under the influence of hormones (e.g. prolactin and oxytocin), milk is produced especially after child delivery. Prolactin is a hormone secreted by the lactotropic cells in the anterior pituitary. It stimulates the growth of the mammary glands and lactation after parturition. It stimulates the mammary glands to produce milk. Oxytocin, in turn, is produced chiefly by the magnocellular neurosecretory cells of the paraventricular nucleus of the supraoptic nucleus of the hypothalamus. Then, it is stored in the posterior pituitary. When needed, oxytocin is released into the circulation in order to reach target cells where it exerts its effects, such as the stimulation of nipples (for milk ejection). It stimulates the muscles around the alveoli to squeeze the milk through the milk ducts.
During milk production, some of the glucose molecules are converted into galactose so that there would more galactose to combine with glucose to produce lactose. N.B.: galactose may be obtained as well from dietary sources. The de novo synthesis of glucose and galactose in the mammary gland is called hexoneogenesis.
Metabolic disorders
In some individuals, the production of lactase decreases with maturity. Others maintain the production of this enzyme and thus remain capable of digesting lactose. Conversely, in the absence, or inadequacy, of lactase, lactose cannot be digested into simpler monosaccharides and as such, leads to lactose intolerance.
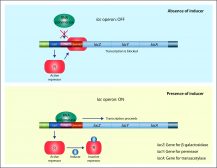
People who are lactose intolerant cannot digest or break down lactose. Lactose that is undigested in the small intestine moves to colon where the gut bacteria ferment it to lactic acid. As a result, methane and hydrogen gas are produced and cause discomfort, gut distention, and flatulence. Diarrhea ensues as water is drawn in to the intestine by the osmotically active lactic acid. Microorganisms, such as Escherichia coli, can metabolize lactose by producing β-galactosidase from its lac operon system.
Biological importance/functions
Lactose is produced naturally and is present in milk of mammals, including humans. It is collected from bovine to be used in preparing infant formulas. A cow’s milk, in particular, has about 4.7% lactose. As of now, there is no infant formula that can match breast milk. Breast milk remains the best provider of vitamins, minerals, hormones, and digestive enzymes. It also has suitable amounts of carbohydrates, proteins, and fats.
Apart from milk, dietary lactose is also present in dairy products. Lactose is a vital dietary carbohydrate since it has a low glycemic index, which means it does not cause spikes in blood sugar level. Just as the other dietary disaccharides, lactose is an important energy source. Hydrolysis of lactose provides simple sugars (galactose and glucose) that the body readily absorbs and metabolizes. Glucose, for instance, is essential since it is favored for use in energy metabolism. It is the form of monosaccharide that the cell commonly uses to synthesize ATP via substrate-level phosphorylation (glycolysis) and/or oxidative phosphorylation (involving redox reactions and chemiosmosis).
Lactose can be converted to lactic acid. Microorganisms, such as Lactobacilli, can convert lactose to lactic acid, which is used in the food industry, e.g. in the production of dairy products like yoghurt and cheese.
Supplementary
Etymology
- Latin lac (“milk”) + –ose (a suffix used in chemical naming of sugars)
IUPAC
Chemical formula
- C12H22O11
Synonym(s)
Derived terms
- Lactose carrier protein
- Lactose factors
- Lactose intolerance
- Lactose permease
- Lactose repressor
- Lactose synthase
- Lactose-litmus agar
Further reading
Compare
See also
Mention(s)
- Breath analysis test
- Dulcite
Reference
- Pasteur, L. (1856). “Note sur le sucre de lait” Note on milk sugar. Comptes rendus (in French). 42: 347–351. From page 348: Je propose de le nommer lactose. (I propose to name it lactose.)
- Berthelot, Marcellin. (1860). Chimie organique fondée sur la synthèse. Mallet-Bachelier (publisher). p.248. Retrieved January 24, 2019, from Link
- Food and nutrition bulletin – Volume 17, Number 4, December 1996. (2019). Retrieved from Link
© Biology Online. Content provided and moderated by Biology Online Editors