Monomer
n., plural: monomers
[ˈmɑnəmɚ]
Definition: the simplest unit, or the repeating unit, of a polymer
Table of Contents
Monomer Definition
What is a monomer? Monomers can be defined as building blocks of a polymer, which is a structure consisting of a number of monomers together.

What is a monomer unit? A monomer unit and monomer have the same meaning. We can define “monomer” in biology as any of those organic compounds that can react with other similar compounds to form a very large molecule (known as a polymer).
Monomer examples: Some monomer examples of biological monomers include monosaccharide molecules that join together to form a carbohydrate polymer. Thus monomer of carbohydrates is a monosaccharide such as glucose or fructose molecules.
What is the monomer of a protein? Similarly, an amino acid is a monomer that forms a complete protein molecule (a polymer). Thus the monomers of proteins are amino acids.
Lipid Monomer: The monomer of lipids are glycerol (a naturally occurring alcohol with a sweet taste) and fatty acids (the building block of fats in our body). The polymers of lipids include diglycerides and triglycerides.
Classification
There are different types of monomers. They can be synthetic monomers, natural monomers, bio monomers, etc. The two broad classes of monomers are based on the type of polymer they form:
- Condensation polymerization monomers
- Addition polymerization monomers
Other classifications of monomers include:
- Linear vs. cyclic (such as ethylene glycol and ethylene oxide respectively)
- Nonpolar vs. polar monomers (ethylene vs. vinyl acetate respectively)
- Synthetic vs. natural monomers (Tetrafluoroethylene and lipid respectively)
Synthetic monomers
Synthetic monomers are artificially made by reacting to different atoms. Once a monomer is formed, it is reacted with other monomers to form a larger synthetic polymer. Synthetic polymers have several uses such as packaging material (polyethylene), binders’ epoxies, and specialized polymers for water treatment. Synthetic monomers are produced using chemical reactions.
Let us define monomers in related fields. In chemistry and biochemistry, a monomer is a molecule that has the ability to react with other monomers to form long-chain polymers. The monomer structure and its binding ability (bi-functional or multifunctional) define whether the resulting polymer will be a single chain type or a three-dimensional complex structure.
Biopolymers
Biopolymers are natural polymers present in living organisms. The monomer unit of biopolymers is connected by covalent bonds forming larger molecules.
The three main classes of biopolymers are:
- Polypeptides and proteins are a polymer made up of amino acid monomers
- Polynucleotide, such as DNA, consists of nucleotides as the monomer unit
- Polysaccharides, such as carbohydrates (starch, alginate, and cellulose), made up of saccharide (sugar) units
Natural rubbers are also an example of a polymer formed from several monomers including suberin, lignin, cutan, cutin, and melanin.
Natural monomers
Natural monomers are building blocks of biopolymers present in nature and can be extracted. The monomers are often water-based. The natural monomer exists in living beings or is produced by cells of such species. Some of the common natural monomers are following:
● Amino acids
Amino acids are the monomers of proteins. The fundamental structure of each amino acid is the same i.e. a central carbon atom bonded with an amino group (NH2), a hydrogen atom, and a carboxyl group (COOH). The structure is shown below:
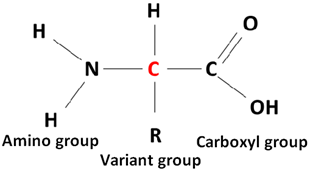
A variant group (R) can be another atom or a group of atoms bonded to the carbon atom to make the polymer. The nature of the variant group bonded to the carbon atom determines the type of amino acid. There are 20 different types of amino acids present in a protein defined by the R group attached to them. The shape, size, function, and other properties of a protein depend on the sequence and number of amino acids.
Each amino acid is connected to another amino acid via a covalent bond (known as a peptide bond). More number of these amino acids connect to form polypeptides (a polymer).
● Nucleotides
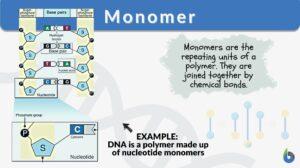
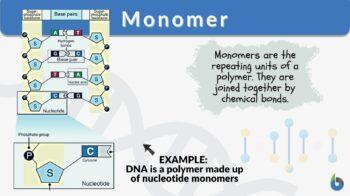
Nucleotides are chemicals that are bonded together to form nucleic acids (RNA or DNA). A nucleotide is a monomer of long chains of nucleotides. A DNA or RNA is a polymer made of these long chains of nucleotides. Nucleotide comprises a sugar molecule (deoxyribose in DNA or ribose in RNA) attached to a nitrogen-containing base and a phosphate group. There are different bases such as in RNA the base consists of uracil, adenine, guanine, and cytosine. In DNA, thymine takes place of uracil.
A structure of nucleotide in DNA is shown in the following figure:
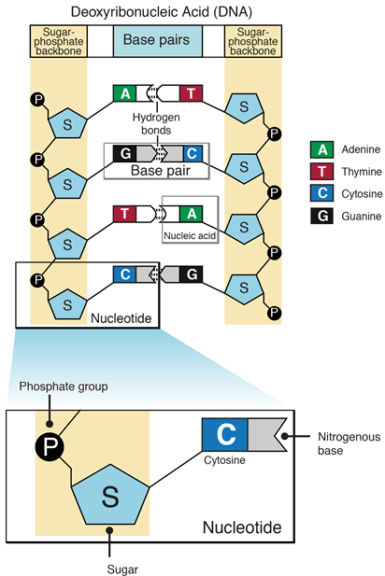
What are the monomers of DNA? The monomers of nucleic acid are nucleotide molecules. Thus nucleic acids monomer are nucleotides.
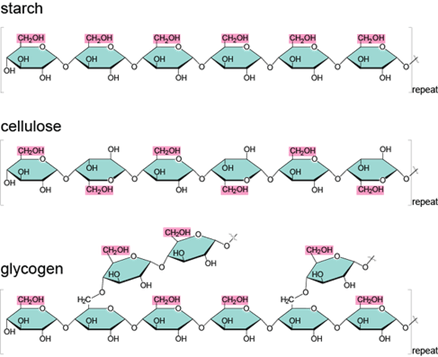
● Glucose and related sugars
In nature, the abundantly existing monomer is glucose. Glucose forms various polymers such as carbohydrates, starch, cellulose, and glycogen. Carbohydrates are one of the simpler forms of a polymer of glucose monomer and are easily digestible. The other polymers are categorized as complex polymers of glucose. Glycosidic bonds are used to connect glucose molecules. It is a type of covalent bond. See how glucose bonds are linked together to form different types of polymers:
● Isoprene
Isoprene is another monomer that occurs in nature. The polymer of isoprene is natural rubber. The structure of isoprene polymer is shown below:
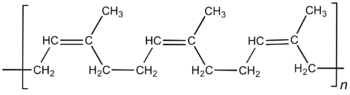
Many plants and animals produce isoprene including humans. A normal human weighting 70kg produces about 17 mg/day of isoprene. It is exhaled from the body using the respiratory tract. Plants produce isoprene polymer i.e. natural rubber. It helps plants to combat environmental stresses such as moderate heat.
A monomer is a molecule that may react chemically to another molecule of the same type to form a larger molecule, such as dimer, trimer, tetramer, polymer, etc. Examples of monomers are amino acids that link together by peptide bonds, forming a polypeptide or a protein. Etymology: from Greek mono “one” and meros “part”. Compare: polymer
Try to answer the quiz below to check what you have learned so far about monomers.
References
- Arrousse, N. (2017). Amino Acid Structure. Retrieved February 18, 2022, from https://www.researchgate.net/figure/amino-acid-structure_fig1_346573462
- NIH. (2019). Nucleotide. Retrieved February 18, 2022, from https://www.genome.gov/genetics-glossary/Nucleotide
Socratic. (2017). What does polysaccharide mean? Retrieved February 18, 2022, from https://socratic.org/questions/599a3c0e7c01493de6f3bdd5 - Socratic. (2018). What is the relationship between monomers and polymers? Give an example using proteins. Retrieved February 18, 2022, from https://socratic.org/questions/what-is-the-relationship-between-monomers-and-polymers-give-an-example-using-pro
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.