Myoblast
n., plural: myoblasts
/ˈmaɪoʊˌblæst/
Definition: A precursor cell that differentiates into a muscle fiber
Table of Contents
Myoblast Definition
A myoblast is a progenitor cell that when it stops dividing enters myogenesis to develop into a myocyte (muscle cell) of the skeletal muscles. Thus, it is a fundamental cell type essential to the development and maintenance of skeletal muscle tissues. During early embryonic development, the myoblasts may divide to produce additional myoblasts especially when there are ample amounts of growth factors. Some of them will stop dividing and differentiate into multi-nucleated myotubes, which later become the muscle fibers, particularly, when there is an insufficient amount of growth factors.
Myoblast Characteristics
How are myoblasts different from other somatic cells? Myoblasts are characterized by the following key features:
- Origin: Myoblasts embryonically originate from somites, which are segments of the paraxial mesoderm. In adults, they originate primarily from satellite cells (stem cells residing in skeletal muscle tissues)
- Morphology: Myoblasts are typically small mononucleated cells with scant cytoplasm; thus, with high nucleus-to-cytoplasm ratio.
- Commitment: Myoblasts have a high capacity for myogenic differentiation. Uninucleated myoblasts differentiating into muscle cells fuse to form long cylindrical striated multinucleated skeletal muscles.
Biology definition:
A myoblast is a stem cell or a progenitor cell responsible for skeletal muscle tissue formation and repair. Etymology: The term “myoblast” is derived from the Greek words “myo,” meaning muscle, and “blastos,” meaning germ or bud.
Skeletal Myogenesis
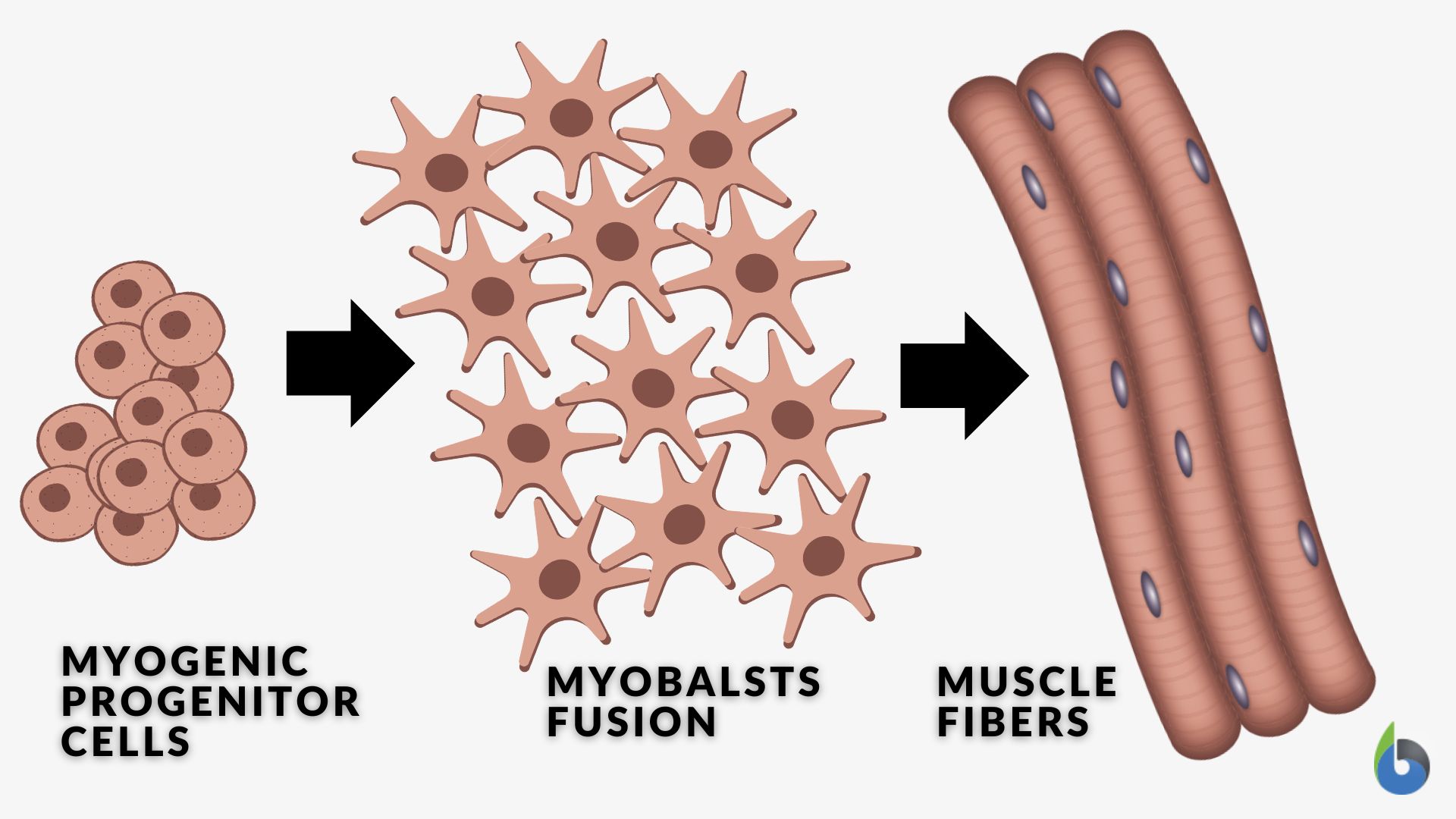
Skeletal myogenesis is a biological process that entails the formation and development of the skeletal muscles, particularly from the myoblasts into mature muscle fibers. During the differentiation stage, certain genes are expressed and the myoblasts align with one another to fuse. Here are the general steps of the complex process of myoblast differentiation.
- During embryonic development, myogenic progenitor cells commit to the myogenic lineage. Cells committed to the myogenic lineage aggregate to form structures called myotomes from where myoblasts will arise.
- Myoblasts express and activate myogenic regulatory factors (MRFs), which are transcription factors that play a pivotal role in regulating muscle-specific gene expression. Examples of MRFs are MyoD (myoblast determination factor), Myf5 (Myogenic Factor 5), Myogenin (Myogenic Factor 4), and MRF4 (Muscle Regulatory Factor 4).
- Myoblasts express and activate muscle-specific genes, such as genes that code for striated alpha-actin and other muscle-specific proteins essential for muscle function.
- Myoblasts cease dividing to specialize from proliferating into differentiating by combining into multinucleated myotubes (the early precursors to mature muscle fibers) with the recruitment of actin to the plasma membrane.
- Within the myotubes, sarcomeres (the contractile units of muscle) begin to form. The myotubes fuse together to form mature muscle fibers.
Myoblast fusion to form skeletal muscle fibers (by Walter Jahn):
Myoblasts In Muscle Regeneration
Myoblasts that did not fuse to form mature muscle fibers are thought to remain in the quiescent state. They become incorporated into mature skeletal muscle during embryonic development. They, then, become a population of myoblasts called satellite cells situated between the sarcolemma and the basal lamina that surrounds the connective tissue. These skeletal myoblasts remain in a quiescent state in adult skeletal muscle until they are needed for muscle regeneration or repair.
When skeletal muscles are injured or damaged, these satellite cells become activated. They enter a proliferative state to generate myoblasts. After successive rounds of proliferation, the myoblasts exit the cell cycle and differentiate into muscle cells by fusing with each other to form new muscle fibers or combining with the existing muscle fibers. This repair mechanism is essential for recovering from injuries, maintaining muscle health, and adapting to increased muscle demand through exercise.
NOTE IT!
Alternative Cell Fates of the Myoblasts?
Myoblasts, primarily, become another myoblast by cell division or a skeletal muscle cell by cell differentiation. Is it always this linear? Can myoblasts differentiate into other mature cells apart from skeletal muscle cells, like smooth muscle cells or cardiac muscle cells?
Smooth muscle cells and cardiac muscle cells originate primarily not from the myoblasts but from another developmental lineate. In particular, the smooth muscle cells come from the mesenchymal cells whereas the cardiac muscle cells are from the cardiogenic mesodermal cells during embryonic development.
Myoblasts that undergo transdifferentiation or adopt alternative fates have been observed although not in normal conditions. Myoblasts differentiating into other cell types was observed in atypical microenvironments (e.g., ectopic differentiation) or through experimental manipulations that promote a different lineage fate (e.g., myoblasts induced to become adipocytes that store fat or fibroblasts that synthesize collagen) under specific conditions.
References
- Birbrair, Alexander; Zhang, Tan; Wang, Zhong-Min; Messi, Maria Laura; Enikolopov, Grigori N.; Mintz, Akiva; Delbono, Osvaldo (2013). “Role of Pericytes in Skeletal Muscle Regeneration and Fat Accumulation”. Stem Cells and Development 22 (16): 2298–314.
- Yaffe, David; Feldman, Michael (1965). “The formation of hybrid multinucleated muscle fibers from myoblasts of different genetic origin”. Developmental Biology 11 (2): 300.
- Zammit, P. S. (2017). Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Seminars in Cell & Developmental Biology, 72, 19-32.
- Buckingham, M., & Rigby, P. W. (2014). Gene regulatory networks and transcriptional mechanisms that control myogenesis. Developmental Cell, 28(3), 225-238.
- Cooper, R. N., Tajbakhsh, S., Mouly, V., Cossu, G., & Buckingham, M. (1999). Muscle regeneration in Duchenne muscular dystrophy. Journal of Cell Biology, 147(3), 1469-1480.
- Noden, D. M., & De Lahunta, A. (1985). The embryology of domestic animals: Developmental mechanisms and malformations. Williams & Wilkins. (Classic Reference)
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.