Nucleotide
n., plural: nucleotides
[ˈnjuːklɪəˌtaɪd]
Definition: an organic compound made up of a nitrogenous base, a sugar, and a phosphate group
Table of Contents
Nucleotide Definition
A nucleotide is regarded as the basic building block of nucleic acid (e.g. DNA and RNA). A nucleic acid, in turn, is one of the major groups of biomolecules (the others are carbohydrates, proteins, and amino acids). Nucleic acids are involved in the preservation, replication, and expression of hereditary information. Nucleotides also provide chemical energy in the form of their nucleoside triphosphates. Additionally, they participate in cell signaling and form a second messenger in cellular processes.
Why do DNA strands not move out of the nucleus to be used as a template for protein synthesis in ribosomes? Join us in our Forum: What does mRNA do in protein synthesis? Tell us what you think!
Characteristics of Nucleotides
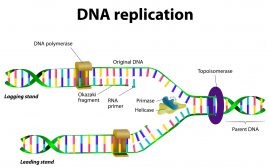
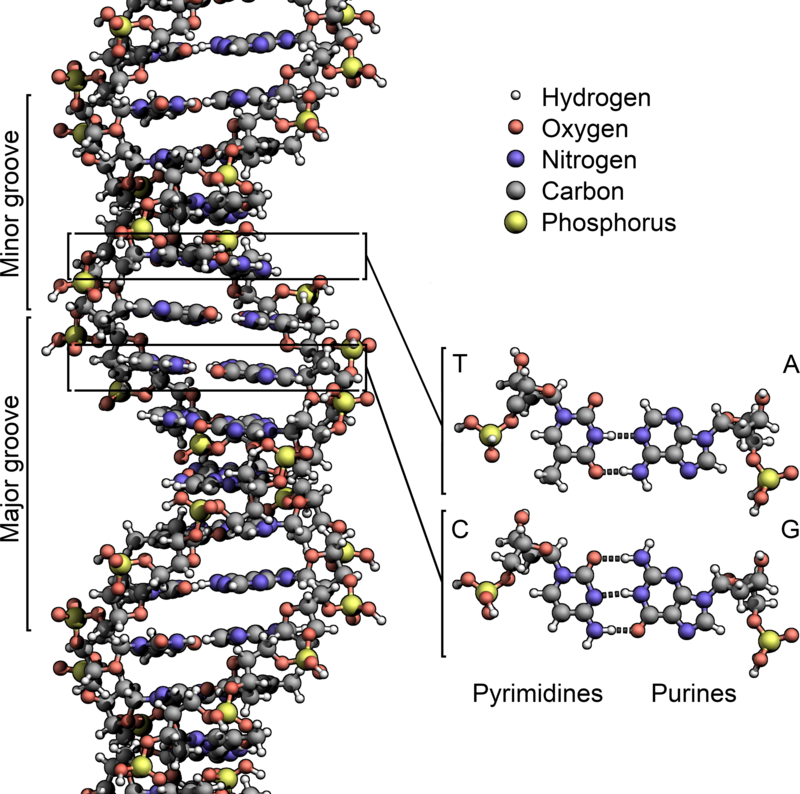
A nucleotide is an organic compound made up of three subunits: a nitrogenous base, a five-carbon sugar, and a phosphate group. The sugar component may either be ribose or deoxyribose. Ribose is the sugar component of the nucleotides that make up RNA. The deoxyribose sugar is the sugar component of DNA. Each phosphate group connects the sugar rings of two adjacent nucleotide monomers. The phosphate groups and the sugar moieties form the backbone of nucleic acid.
In DNA, the orientation of the two strands is in opposite directions. This is to allow complementary base pairing between nucleobase constituents. Apart from the long chain of nucleic acids, nucleotides also occur in cyclic forms. Cyclic nucleotides form when the phosphate group is linked twice to the sugar moiety, particularly to the two hydroxyl groups of the constituent sugar.
Apart from the role of nucleotides as subunits of nucleic acids, they are also energy carriers. They carry chemical energy that the cell uses to fuel various cell activities. Adenosine triphosphate (ATP) is by far the most widely used.
Nucleosides vs. Nucleotides
Nucleotides should not be confused with nucleosides, which are also 5-carbon sugar with a nitrogenous base. Nucleosides do not have a phosphate group. When a nucleoside is bound to a phosphate group, it yields a nucleotide. (Ref. 1) Thus, a nucleotide is also referred to as nucleoside monophosphate (if with only one phosphate group), nucleoside diphosphate (with two phosphate groups), or nucleoside triphosphate (when with three phosphate groups).
Depending on the pentose sugar component, a nucleoside may be a ribonucleoside or a deoxyribonucleoside. A ribonucleoside is a nucleoside with a ribose (sugar component). Based on the nucleobase component, the ribonucleoside may be adenosine, guanosine, cytidine, uridine, or 5-methyluridine. A deoxyribonucleoside is a nucleoside with deoxyribose. Similarly, based on the nucleobase component, a deoxyribonucleoside may be deoxyadenosine, deoxyguanosine, deoxycytidine, deoxythymidine, or deoxyuridine.
Also, depending on the nucleobase component, the nucleosides may be grouped into either the “double-ringed” purine or the “single-ringed” pyrimidine.
Classification of Nucleotides
The fundamental nucleotides are divided into purines and pyrimidines based on the structure of the nitrogenous base. The purine bases include adenine and guanine while the pyrimidine bases are thymine and cytosine, and uracil. In RNA uracil replaces thymine (thymine is produced by adding methyl to uracil). (Ref. 2)
The nucleobases that make up the nucleic acid are used to distinguish DNA from RNA molecules. In DNA, thymine complementary pairs with adenine whereas in RNA, uracil matches with adenine. The pairings of nucleobases C-G and A-T (or A-U in RNA) are referred to as base complements.
Types of Nucleotides
Examples of nucleotides with only one phosphate group:
- adenosine monophosphate (AMP)
- guanosine monophosphate (GMP)
- cytidine monophosphate (CMP)
- uridine monophosphate (UMP)
- cyclic adenosine monophosphate (cAMP)
- cyclic guanosine monophosphate (cGMP)
- cyclic cytidine monophosphate (cCMP)
- cyclic uridine monophosphate (cUMP)
- deoxyadenosine monophosphate (dAMP)
- deoxy guanosine monophosphate (dGMP)
- deoxtcytidine monophosphate (dCMP)
- (deoxy)thymidine monophosphate (dTMP)
Nucleotides with two phosphate groups:
- adenosine diphosphate (ADP)
- guanosine diphosphate (GDP)
- cytidine diphosphate (CDP)
- uridine diphosphate (UDP)
- deoxyadenosine diphosphate (dADP)
- deoxyguanosine diphosphate (dGDP)
- deoxycytidine diphosphate (dCDP)
- (deoxy)thymidine diphosphate (dTDP)
Nucleotides with three phosphate groups:
- adenosine triphosphate (ATP)
- guanosine triphosphate (GTP)
- cytidine triphosphate (CTP)
- uridine triphosphate (UTP)
- deoxyadenosine triphosphate (dATP)
- deoxyguanosine triphosphate (dGTP)
- deoxycytidine triphosphate (dCTP)
- (deoxy)thymidine triphosphate (dTTP)
The sequence of DNA nucleotides in the nucleus is used as a template to synthesize mRNA. Discover more here: What does mRNA do in protein synthesis? Join our Forum now!
De Novo Synthesis Pathway
Nucleotides are produced naturally by de novo synthesis pathway or by salvage pathways. (Ref. 4) In humans, the de novo synthesis pathway of the fundamental nucleotides occurs mainly in the liver. In pyrimidine biosynthesis, the ring forms by a series of steps that begin with the formation of carbamoyl phosphate. (Ref. 1) First, carbamoyl phosphate is produced from a biochemical reaction that involves bicarbonate, glutamine, ATP (for phosphorylation), and a water molecule. The enzyme that catalyzes the reaction is carbamoyl phosphate synthetase II located in the cytosol. Next, the carbamoyl phosphate is converted into carbamoyl aspartate by the enzyme aspartate transcarbamylase. Then, the ring closes through intramolecular condensation, converting carbamoyl phosphate into dihydroorotate by the enzyme dihydroorotase. Lastly, the dihydroorotate is oxidized by dihydroorotate dehydrogenase (an integral membrane protein in the inner mitochondrial membrane) to convert into orotate. After the pyrimidine ring forms, 5-phospho-α-D-ribosyl 1-pyrophosphate (PRPP), a ribose phosphate, reacts to orotate to form orotidine-5-monophosphate (OMP). OMP is then decarboxylated by the enzyme OMP decarboxylase to yield uridine monophosphate (UMP). Eventually, uridine diphosphate (UDP) and uridine triphosphate (UTP) are produced down the biosynthetic pathway by kinases and dephosphorylation of ATPs. UTP can be converted into cytidine triphosphate (CTP) by amination of UTP via the enzyme CTP synthetase. (Ref. 5)
In purine biosynthesis, purines may come from the nucleotide inosine monophosphate (IMP). IMP, in turn, is produced from a pre-existing ribose phosphate that forms mainly from the amino acids glycine, glutamine, and aspartic acid. Ribose 5-phosphate reacts with ATP to produce 5-Phosphoribosyl-1-pyrophosphate (PRPP). PRRP has a role in both purine and pyrimidine synthesis; it is also involved in NAD and NADP formation and salvage pathways. PRRP though becomes committed particularly to purine biosynthesis when PRRP is converted into 5-phosphoribosyl amine (by having the pyrophosphate of PRRP replaced by the amide group of glutamine). (Ref. 6) IMP is then converted into either adenosine monophosphate (AMP) or guanosine monophosphate (GMP).
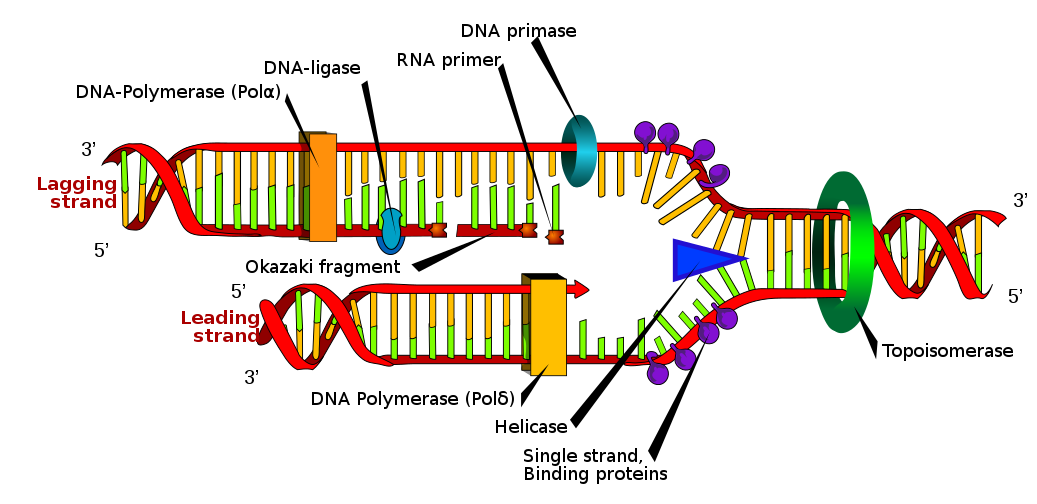
Where is mRNA synthesized? Our Expert gives the answer. Read them here: What does mRNA do in protein synthesis? and more! Come and join us in our Forum.
Degradation
Purines guanine and adenine may be degraded as follows:
As for GMP, the compound is first hydrolyzed and converted to guanosine. The latter is then cleaved to free guanine. (Ref. 7)
- Guanine (via guanase) » xanthine (via xanthine oxidase) » uric acid
- Adenosine »» inosine (via purine nucleoside phosphorylase) » hypoxanthine (via xanthine oxidase) » xanthine (via xanthine oxidase) » uric acid
As a result of purine degradation, uric acid is produced. In humans, uric acid is released from the liver and other tissue sources into the bloodstream through which it reaches the kidney. It is then excreted from the body via urine.
Purines from catabolism may be salvaged and re-used as follows: (Ref. 6)
- Adenine is salvaged by the enzyme adenine phosphoribosyltransferase (APRT), by converting it to adenylate
- Guanine and hypoxanthine are salvaged by the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRT), by forming guanylate or IMP
Pyrimidines that are degraded can be recycled by a salvage pathway. (Ref. 1) Nucleobases are recovered for re-use post-RNA and DNA degradations. Pyrimidine salvage pathways are as follows:
- Cytosine is converted into uracil by deamination. By uridine phosphorylase, uracil is converted into uridine by reacting with ribose-1-phosphate. Through the enzyme nucleoside kinase, uridine is converted into uridine monophosphate (UMP).
- Thymine is converted into thymidine by reacting with deoxyribose-1-phosphate and by the enzyme thymidine phosphorylase. Thymidine is then converted into thymidine monophosphate by the enzyme nucleoside kinase. Thymidine kinase, in particular, is an enzyme of the pyrimidine salvage pathway that catalyzes the phosphorylation of thymidine to thymidine monophosphate. (Ref.8)
Biological Functions of Nucleotides
Aside from serving as precursors of nucleic acids, nucleotides also serve as important cofactors in cellular signaling and metabolism. These cofactors include CoA, flavin adenine dinucleotide (FAD), flavin mononucleotide, adenosine triphosphate (ATP), and nicotinamide adenine dinucleotide phosphate (NADP). The nucleoside triphosphates, in particular, carry packets of chemical energy that are used in many cellular activities demanding energy, e.g. amino acid synthesis, protein synthesis, cell division, internal and intercellular movements, etc.
Reviewed by: Todd Smith, PhD
Try to answer the quiz below to check what you have learned so far about nucleotides.
See also
Recommended:
- BLAST for beginners. Digital World Biology. https://digitalworldbiology.com/tutorial/blast-for-beginners
References
- PURINES AND PYRIMIDINES. (2020). Utah.Edu. https://library.med.utah.edu/NetBiochem/pupyr/pp.htm
- Bera, P. P., Nuevo, M., Materese, C. K., Sandford, S. A., & Lee, T. J. (2016). Mechanisms for the formation of thymine under astrophysical conditions and implications for the origin of life. The Journal of Chemical Physics, 144(14), 144308. https://doi.org/10.1063/1.4945745
- Nucleotides. (2020). Rpi.Edu. https://homepages.rpi.edu/~bellos/nucleotides.htm
- Nucleotide Salvage – an overview | ScienceDirect Topics. (2018). Sciencedirect.Com. https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/nucleotide-salvage
- Charma, K. & Somani, D. (2015). Pyrimidine Biosynthesis. Retrieved from Slideshare.net website: www.slideshare.net/kskuldeep1995/pyrimidine-biosynthesis-46874172
- Berg, J. M., Tymoczko, J. L., & Lubert Stryer. (2016). Key Steps in Nucleotide Biosynthesis Are Regulated by Feedback Inhibition. Nih.Gov; W H Freeman. https://www.ncbi.nlm.nih.gov/books/NBK22428/
- Chapter 21 : Biosynthesis of Amino Acids, Nucleotides, and Related Molecules. (2020). Bioinfo.Org.Cn. http://www.bioinfo.org.cn/book/biochemistry/chapt21/bio8.htm
- He, Q., Mao, Y., & Wu, J. (2002). Immunohistochemical Expression of Cytosolic Thymidine Kinase in Patients with Breast Carcinoma. Handbook of Immunohistochemistry and in Situ Hybridization of Human Carcinomas, 463–469. https://doi.org/10.1016/s1874-5784(04)80056-4
© Biology Online. Content provided and moderated by Biology Online Editors