![Osmotic pressure n., plural: osmotic pressures [ɑsˈmɑtɪk ˈpɹɛʃ.ɚ] osmotic pressure definition and example](https://www.biologyonline.com/wp-content/uploads/2020/11/osmotic-pressure-definition-and-example-350x196.jpg)
Osmotic pressure
n., plural: osmotic pressures
[ɑsˈmɑtɪk ˈpɹɛʃ.ɚ]
Definition: pressure exerted by a solution against a biological membrane
Table of Contents
Osmotic Pressure Definition
Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. Osmotic pressure reduces water potential, which is the tendency of water to move from one area to another.
Osmotic pressure is essential for many living things. For plants, it is vital in plant turgidity (by providing support in plant cells) and for osmoregulation. In the food industry, the principle behind osmosis and osmotic pressure is used in fruit preservation. Sugar is often used to preserve food as its addition causes water to be drawn out from cells, including microbial cells. Hence, these microbes cannot grow and will die out from losing water as the water is drawn out by osmosis.
Osmotic Pressure and Hydrostatic Pressure
Is osmotic pressure and hydrostatic pressure the same? It can be confusing as some references say that they’re the same and one. Others are more stringent in their definition and others still, regard them as opposites. Etymologically, the word “hydrostatic” means “water” (hydro) and “not flowing” or “at rest”(static). Thus, hydrostatic pressure is generally defined as the pressure exerted by water (or any fluid) at rest or when it’s not flowing in a confined space. The pressure is caused by the force of gravity. Therefore, the factors that affect hydrostatic pressure are the density of the liquid, the depth of the water (or fluid) column, and the acceleration of gravity. For instance, the greater the water density or the deeper the water column is, the higher the hydrostatic pressure will be. Osmotic pressure is defined above as the pressure needed to stop the flow of water, thus the two are construed as the same in some references. (Osmosis, Tonicity, and Hydrostatic Pressure, 2023)
Conversely, there are also many sources that regard the two as different terms, especially when we speak of pressures in blood vessels, capillaries, and interstitial fluid. (Capillary Exchange | Anatomy and Physiology II, 2023)
In particular:
- Blood hydrostatic pressure (BHP), specifically, is the term used to refer to the pressure that is exerted by the blood (“pushing”) against the wall of blood vessels.
- Capillary hydrostatic pressure (CHP) is the term used to refer to the pressure exerted by blood against the wall of capillaries
- Interstitial fluid hydrostatic pressure (IFHP) is the pressure of the interstitial fluid
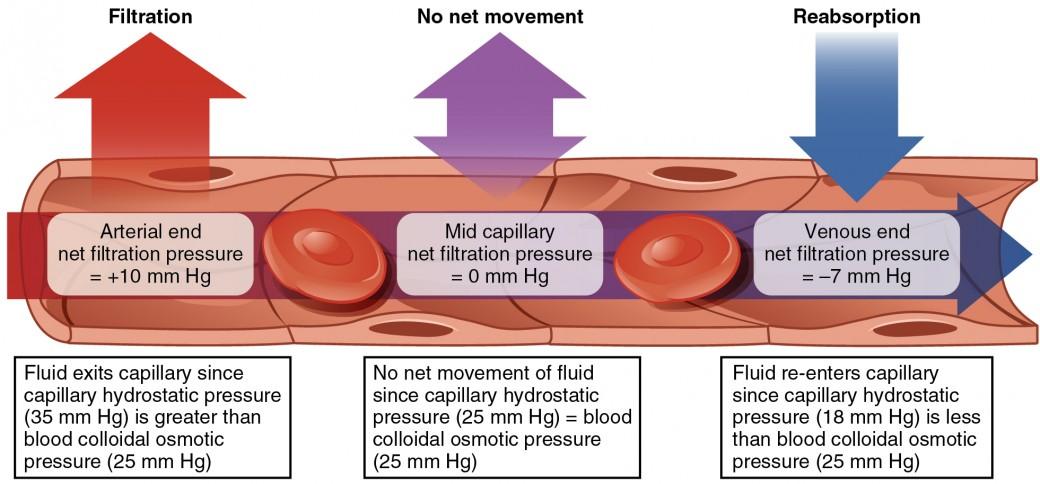
The capillaries are the site of fluid exchange mediated by hydrostatic pressure and osmotic pressure. Because of BHP and CHP, the fluid is drawn out of the blood vessels and capillaries, respectively, into the tissue, causing the IFHP in the interstitial fluid to rise.
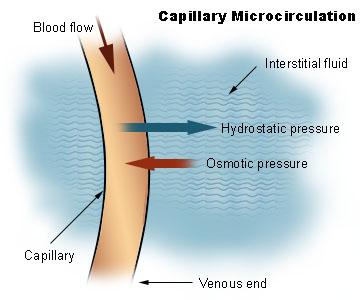
Osmotic pressure is the pressure that tends to “pull” the fluid back into the capillaries, working opposite of the hydrostatic pressure. Osmotic pressure is also sometimes called “oncotic pressure”. Osmotic pressure, in this sense, becomes different from hydrostatic pressure. Osmotic pressure arises from the differences in solute concentration and not from gravity.
The molecules that create osmotic concentration gradients in the blood are the plasma proteins and not the salts and the formed elements in the blood. The pressure caused by the presence of colloidal proteins in the blood is called blood colloidal osmotic pressure (BCOP) as opposed to the interstitial fluid colloidal osmotic pressure (IFCOP), the pressure caused by the colloidal proteins in the interstitial fluid.
Because the colloidal osmotic pressure is higher in blood than in the interstitial fluid (i.e., BCOP > IFCOP), the water tends to be drawn from the tissue back into the capillaries (particularly at the venule end of the capillary), which in this case is called “reabsorption”.
Turgor Pressure vs. Osmotic Pressure
In plant cells, the hydrostatic pressure that is exerted by the fluid against the cell wall is referred to as turgor pressure. Turgor pressure is essential to plants. Without much turgor pressure, the plant cells will not have the “force” that pushes the plant cell membrane against the cell wall, appearing ‘swollen’.
Turgidity, the condition of being turgid (or swollen), is important for plant cells as it renders these cells to be stiff and rigid. Without turgor pressure, the plant cells become “flaccid” and outwardly will be looking “wilted“.
But what makes osmotic pressure different from turgor pressure? Both osmotic pressure and turgor pressure are forces that result due to differences in water and solute concentrations. However, turgor pressure is the pressure of a fluid, such as water, that tends to push the cell membrane against the cell wall. Osmotic pressure, in this regard, refers to the pressure that needs to be used on the solution so that water will be prevented to flow across the membrane. In simpler words, turgor pressure is the pressure inside the plant cell whereas osmotic pressure is the pressure of the water or solvent molecules inside or outside the plant cell.
Now, let’s take a look at the process of water movement through biological membranes — osmosis.
Osmosis and Osmotic Potential
Osmosis refers to the net movement of water molecules across the membrane from areas of higher to lower water potential. The process can be likened to simple diffusion where the movement is downhill (not requiring chemical energy to proceed). However, osmosis looks at the movement of the solvent (water) rather than the solute molecules of a solution across the membrane.
Note it!
By definition, osmosis is the net movement of water molecules through a biological membrane from an area of greater concentration to an area of lower concentration of water molecules separated by a semipermeable membrane. The tendency of the water molecules to move from one area to another is called “water potential“, and if it occurs by osmosis, then it is referred to as “osmotic potential” (as opposed to others causing water potential, such as gravity).
The principle of osmosis is used in reverse osmosis where water molecules are filtered artificially by forcing them to pass across a semipermeable membrane. However, rather than passively, as in natural osmosis, water moves actively, in the other direction (thus, reverse).
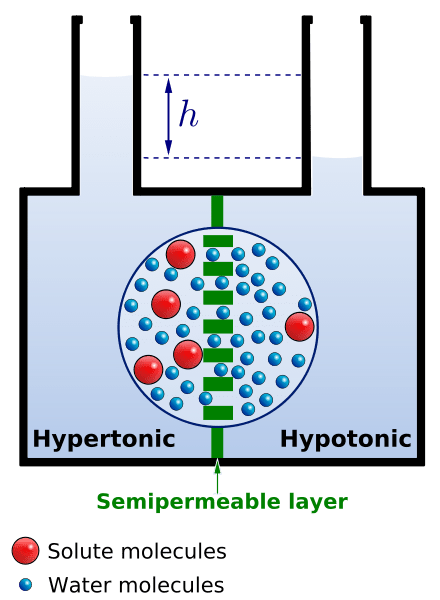
Osmotic potential is the potential of water molecules to move from one solution to another across the membrane. If you will remember, a solution consists of two components: the solute and the solvent.
Depending on the solute and solvent components, a solution may be:
- Isotonic solution, where two solutions are of the same solute concentrations
- Hypotonic solution, where one solution has less solute and more water than the other solution
- Hypertonic solution, where one solution has more solute and less water than the other solution
Osmotic concentration (or osmolarity) refers to the measure of solute concentration (number of osmoles of solute per liter). It can be used to determine what type of solution it is, if isotonic, hypotonic, or hypertonic.
Pure water (pure solvents) lacks solutes and thus, when used as a reference against any solution of the same volume, the water potential of the latter will always be less than that of pure water.
Two isotonic solutions would tend to have no net water movement. But in the case of hypertonic (higher solute concentration) and hypotonic (lower solute concentration) solutions, what do you think will happen? Water will move from hypotonic to hypertonic solution — or from the region of higher osmotic potential to the region of lower osmotic potential.
Osmotic Pressure Formula
We already defined osmotic pressure as the pressure caused by the pressure difference and in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. But more than that, it can also be expressed mathematically. In essence, osmotic pressure will be the minimum pressure needed to stop the flow of water molecules across the semipermeable membrane. It can be calculated using the following equation:
π = iCRT
i.e.,
osmotic pressure (π) = van’t Hoff factor (i) * molar concentration of the solute in the solution (C) * ideal gas constant (R) * temperature (T)
This equation thus shows that osmotic pressure is a colligative property of a solution. It depends on the ratio of the number of solute particles to the volume of the solution, not on the identity of the solute particles. (Colligative Properties, 2023) Other related colligative properties of solutions include the freezing point depression, the boiling point elevation, and the vapor pressure depression. (Feher, 2017)
Types of Osmotic Pressure
Essentially, there are three types of osmotic pressure according to solute and solvent components:
- Isoosmotic pressure, where two solutions are of the same solute concentrations, thus, with the same or equal osmotic pressure
- Hypoosmotic pressure, where a solution is characterized by having a lower osmotic pressure than a surrounding fluid under comparison
- Hyperosmotic pressure, where a solution is characterized by having a higher osmotic pressure than a surrounding fluid under comparison
Examples
In the kidneys, there are varying osmotic pressures:
- At the beginning of the loop of Henle: the tubular fluid is isoosmotic to plasma (surrounding fluid)
- At the tip of the loop of Henle: the tubular fluid is hyperosmotic to the plasma
- When it leaves the loop of Henle: the tubular fluid is hypoosmotic to the plasma
Another example is the response of living cells to different osmotic environments. See the Figure below to see how the cells of a fresh water fish would react if placed into a container with:
(1) a hypoosmotic environment (with less solute, more water, higher water potential, and lower osmotic pressure than the cells)
(2) an isoosmotic environment (same solute/water concentration/osmotic pressure)
(3) a hyperosmotic environment (with more solute, less water, lower water potential, higher osmotic pressure than the cells)

Watch this vid about osmotic pressure:
Take the quiz on Osmotic Pressure!
References
Osmosis, Tonicity, and Hydrostatic Pressure. (2023). Colostate.edu. http://www.vivo.colostate.edu/hbooks/pathphys/topics/osmosis.html#:~:text=Osmotic%20pressure%20is%20defined%20as,or%20a%20layer%20of%20cells.
Capillary Exchange | Anatomy and Physiology II. (2023). Lumenlearning.com. https://courses.lumenlearning.com/suny-ap2/chapter/capillary-exchange/
Colligative Properties. (2023). Purdue.edu. https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch15/colligative.php
Feher, J. (2017). Quantitative Human Physiology: An Introduction. Elsevier Inc. https://www.sciencedirect.com/book/9780128008836/quantitative-human-physiology
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.