Osteocyte
n., plural: osteocytes
[ˈɒstɪəʊˌsaɪt]
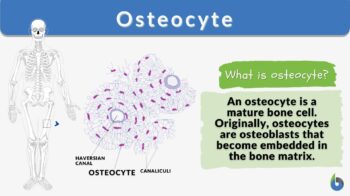
Definition: a mature bone cell
Table of Contents
Osteocyte Definition
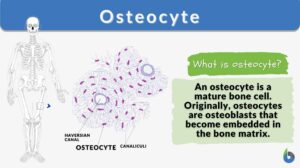
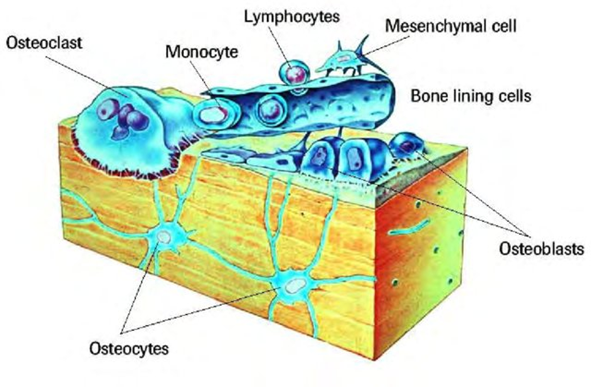
The osteocyte is a mature bone cell. Other bone cell types are osteoprogenitor cells, osteoblasts, and osteoclasts. Osteoprogenitor cells give rise to active osteoblasts. Osteoblasts are bone-forming cells whereas osteoclasts are degradative cells that break down and reabsorb bone.
During bone formation, the osteoblasts secrete materials that make up the bone matrix, and as they secrete them some of them are eventually trapped and buried in the bone matrix. Osteoblasts that have become surrounded by the bone matrix are referred to as osteocytes. As osteocytes may have appeared ‘entombed’ in the mineralized matrix, they are not dead cells.
Osteocytes have some interesting features. For instance, osteocytes can live long, depending on the skeletal age. Unlike other bone cells like osteoblasts and osteoclasts that are short-lived, osteocytes live relatively long and they don’t divide. Aged osteocytes may die eventually from senescence.
In humans, the osteocytes typically live through the human lifespan but some of them die from aging, typically after reaching menopausal age. Pathological conditions can also lead to bone tissue death (osteonecrosis).
The only place where the osteocytes develop is mesenchyme, which is the loosely connected embryonic tissue that produces most of the body’s connective tissues.
Watch these videos about osteocytes, osteoblasts, and osteoclasts:
Biology definition:
An osteocyte is a mature bone cell. It is a stellate, non-dividing cell embedded in mature bony tissue. Apart from the osteocytes, the other main types of bone cells are osteoclasts, osteoblasts, and lining cells.
These bone cells are responsible for forming the bones (replacing the cartilage), and thus, the skeleton of vertebrates.
Etymology: osteo- (“bone”) + -cyte (“cell”)
See also: osteoblast, bone
Osteocyte Structure
Let’s now take a look at the osteocyte structure (see bone cell diagram below).
Stellate (Star-like) shape
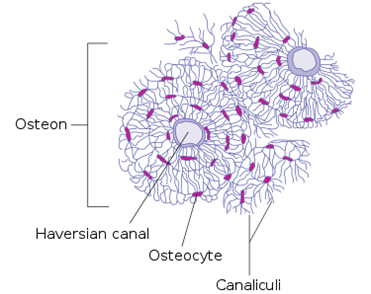
Osteocytes are stellate in shape. The stellate shape is due to the presence of cytoplasmic extensions (cell processes) that radiate towards the mineralizing matrix. The cell processes of osteocytes occupy the canaliculi and connect to each other. The cell processes are used for the exchange of nutrients and waste via the gap junctions.
The cell processes radiate primarily towards the bone surface in circumferential lamellae, or towards a Haversian canal and outer cement line that are characteristic of osteons in concentric lamellae bone. In the mineralized type I collagen matrix, osteocytes generate a huge lacuna-canalicular network, with the assistance of cell bodies dwelling in lacunae and cell/dendritic processes residing in canaliculi.
Cell size
Typically, an osteocyte may be around 7 micrometers deep and 15 micrometers broad in length. The diameter of the cell body can range anywhere from 5 to 20 micrometers, and it can include 40 to 60 cell processes. The space between inert cells can range from 20 to 30 micrometers.
Osteocyte organelles
An osteocyte that has reached maturity contains a single nucleus (mononucleated). The orientation of the nucleus towards the vascular side. Furthermore, it has one or two nucleoli and a membrane. In addition, a smaller Golgi apparatus, mitochondria, and endoplasmic reticulum are osteocyte organelles.
Evolutionary Origins and Development
Bone formation is one of the distinctive characteristics of vertebrates. However, little is known about the evolutionary origins of the bone cells, including osteocytes. Nevertheless, osteocytes, in particular, are important in paleontology as these cells have a reliably preserved shape in the lacunae of bone fossils.
250 to 400 million years ago, osteocytes were found in fish (jawless types), according to historical data. It has been demonstrated that osteocyte size and genome size are correlated and paleogenomics research has utilized this correlation of sizes. Osteostracans (early jawless vertebrates) are the sister group to jawed vertebrates, gnathostomes and their last common ancestor could have been the first to evolve osteocytes. (Haridy et al., 2021)
An essentially osteoblasts surrounded osteoblast becomes enclosed in the bone matrix during bone formation as an “osteoid osteocyte” connects to other osteoblasts through a complex chain of biological events. Matrix metalloproteinases (MMPs), osteoblasts and osteocytes factor 45 (OF45), dentin matrix protein 1 (DMP-1), Klotho, lysophosphatidic acid (LPA), E11 antigen, TGF-beta inducible factor (TIEG), and healthy osteocytes are produced in the proper numbers or in certain distributions, all of which are dependent on adequate levels of oxygen. Ten to twenty percent of osteoblasts differentiate into osteocytes.
From osteoblast to mature osteocytes cells, Palumbo and his team 1990 separate three bone cell types:
Bone cell types | |
|---|---|
| Types (preosteocyte) | Names |
| Type I | osteoblastic osteocyte |
| Type II | osteoid osteocyte |
| Type III | partially surrounded by a mineral matrix |
Data Source: Shoaib Zaheerof Biology Online
It takes about three days for an osteoblast to transition into an entrapped osteocyte. In three days, three times as much extracellular mineralized bone matrix is synthesized by the cell. As a result, the mature osteocyte’s cell body is 70% reduced in volume than that of the osteoblast.
Now the cell transforms into a radical shape from the polygonal shape. The dendrites move towards the mineralizing front and then towards the vascular or bone surface. Alkaline phosphatase levels decrease while the levels of osteocalcin and casein kinase II increase during the transformation of an osteoblast into an osteocyte.
Osteoid-osteocytes must break collagen and other matrix-embedded cell components to control mineralization and create connective dendritic processes.
They seem to have more bone morphogenetic proteins that can tolerate hypoxia since they are entrenched in the bone lining cells and get very little oxygen. The reason for this is unclear. Oxygen tension may be responsible for controlling the growth of osteoblasts into osteocytes and disuse-induced bone resorption may be mediated by hypoxia in osteocytes.
Osteocytes in Osteogenesis and Bone Remodeling
How do osteocytes form? Osteocytes form as part of the process of osteogenesis (bone formation and development). There are two major modes of osteogenesis — intramembranous ossification and endochondral ossification. In intramembranous ossification, the mesenchymal tissue is converted directly to a bone (direct ossification).
In endochondral ossification, the mesenchymal cells first differentiate into chondrocytes, the cells that form the cartilage, and later the cartilage is replaced by bone (indirect ossification).
In both modes of osteogenesis, the mesenchymal cells are the source of either chondrocytes or osteocytes. Mesenchymal cells are stem cells from where many types of cells come from and are mainly located in the bone marrow.
During the early development, some of them can later develop into either chondrocytes or osteoblasts depending on the signaling molecules they receive, and such mesenchymal stem cells are referred to as osteochondroprogenitor cells. So for instance, Sox9, L-Sox5, and Sox6 are signaling molecules that help them differentiate particularly into chondrocytes and Runx2 and Osx are signaling molecules that signal them to differentiate into osteoblasts.
Note though that osteochondroprogenitor cells undergo cellular changes to become osteoprogenitor cells committed to developing into osteoblasts. These cells become larger and form more Golgi apparatus and rough ER until they develop morphologically into osteoblasts.
Active osteoblasts, the bone-forming cells, act as groups (osteons) and connect with each other to form the bone matrix (osteoid). They secrete mainly collagen and a few other proteins (e.g. osteocalcin and osteopontin), which serve as the initial components of an organic bone matrix. Later, they secrete calcium phosphate, which hardens and forms the mineral, hydroxyapatite in the bone matrix.
Osteoblasts that become trapped in their own secretions become the osteocytes. The osteocytes are no longer mitotically active (thus, have undergone osteocyte differentiation) and have reduced synthetic activity. Nevertheless, they are actively involved in osteocytic osteolysis. They are important in the regulation of bone mass, acting as mechanosensor cells that control the activity of osteoblasts and osteoclasts during bone remodeling.
Osteocytes Function
Even though osteocytes are not very active cells, they can perform molecular synthesis and modification, in addition to the sending of messages across vast distances, in a pattern that is analogous to that of the nervous system and beyond the bone microenvironment. They make up the majority of the bone’s cell population (31,900/ mm3 to 93,200/ mm3 from bovine cortical bone to transgenic mice bone respectively).
The mature osteocyte network has the vast majority of the receptor functions that contribute significantly to the proper functioning of bone physiology. In particular, osteocytes have been known to play in mineral homeostasis, mechano-sensing, and orchestrating bone remodeling.
Osteocyte regulation has an important part to play in the control of bone mass. A transfer system that can sense and have all information is indicated by the presence of glutamate transporters in osteocytes, which are responsible for the production of nerve growth factors followed by bone fracture.
An increase in bone resorption, a decrease in bone mineral density, a trabecular bone loss, and a loss of responsiveness to unloading were all observed in the bones after an experiment in which osteocytes proteins were selectively removed.
In a multicellular organism, osteoblasts and osteoclasts are controlled by mechanosensor cells. Throughout this, the bone undergoes a process known as remodeling bone. Osteocytes are responsible for producing an inhibiting signal, which is then communicated to osteoblasts via the processes that occur within their cells to facilitate bone growth.
Osteocyte proteins produce crucial endocrine cell regulators of the phosphate and mineral metabolism of the bone. Sclerostin and other molecules such as FGF-23, DMP-1, PHEX, and MEPE which regulate phosphate and biomineralization, are highly expressed by osteocytes. They have been identified to operate in mineral metabolism.
The regulation of osteoclasts may be associated with the disease. For example, Lynda Bonewald hypothesized that osteocytes secrete FGF23, which travels via the circulatory system and stimulates the kidneys to release phosphorus. As in X-linked hypophosphatemia, teeth and bones become brittle, and muscles get shaky and feeble, without sufficient phosphorus.
Sclerostin
By attaching to the LRP5/LRP6 coreceptor and suppressing Wnt signaling, sclerostin inhibits bone turnover generated by osteocytes. As a consequence of the SOST gene, sclerostin, the initial mediator of communication between bone-resorbing osteoclasts, osteocytes, and bone-forming cells osteoblasts is essential for bone remodeling.
Only osteocytes produce sclerostin, which acts as a paracrine inhibitor thus it inhibits bone formation. Other major factors are parathyroid hormone and mechanical loading which decreases the sclerostin.
Pathophysiology
Osteonecrosis is a term that describes the typical pattern of cell death as well as the complex bone formation and resorption and osteogenesis. Osteocyte necrosis (ON) begins with the death of hematopoietic and adipocytic cells, in addition to edema in the interstitial spaces of the bone marrow.
ON occurs after around two to three hours of anoxia, although the osteocyte histology markers of necrosis do not manifest until approximately twenty-four to seventy-two hours following hypoxia. Pyknosis of the nuclei is the earliest indicator of osteonecrosis, followed by the presence of hollow osteocyte lacunae.
At the periphery of necrosis, capillary revascularization and reactive hyperemia are very rare. A further healing procedure follows, including both bone resorption and bone formation, to partly revive dead tissue and restore bone homeostasis.
Along with the fragmented resorption of dead bone, the nouveau bone will overlay on top of trabeculae that have died. The human bone resorption rate is quite higher as compared to the bone creation rate, as a result of this inflammatory bone loss, subchondral trabeculae loss of structural integrity, subchondral fractures, and joint incongruity.
Clinical Significance
Human CD34+ stem cells’ osteocytic potential has been modeled in three dimensions. and the research has significant clinical significance. The findings demonstrate that the model exhibit an osteogenic differentiation capability that is unique to them and that they are suitable for usage in the early stages of bone metabolism (repair) following injury.
Necrosis/Degeneration, senescence, apoptosis (programmed cell death), and osteoclastic engulfment are all causes of osteocyte death. In some cases, all four of these processes are involved. Nearly seventy-five percent of bone osteocytes are dead (decreased bone mass) by the age of 80 (age-related bone loss), but it is less than one percent when a person is born.
Osteocytes are hypothesized to undergo apoptosis as a result of diminished mechano-transduction, which may be one of the factors that contribute to the development of osteoporosis. In osteocyte apoptosis, there is a release of apoptotic bodies by the apoptotic osteocyte that express RANKL to recruit osteoclasts.
The viability of osteocytes is enhanced by mechanical stress, which also aids in the movement of solutes via the lacuno-canalicular system in bone, hence improving O2 level and nutrient flow into the osteocytes themselves. It has been demonstrated that skeletal unloading can produce osteocyte hypoxia in vivo. When this happens, osteocytes commit suicide by going through the apoptosis process and recruiting osteoclasts to resorb bone.
Micro damage in the bone structure is caused by recurrent occurrences of loading cycling, and it seems to be connected with the death of osteocytes caused by apoptosis. As the bone surfaces age, the level of TGF- decreases, and the gene expression of osteoclast-stimulatory factors, increases, which increases bone subsequent resorption, leading t bone loss. Osteocytes, under typical circumstances, produce a significant amount of TGF-, which in turn serves to inhibit bone formation.
When osteocytes are mechanically stimulated, the hemi channels that allow the release of PGE2 and ATP, as well as other biochemical signaling molecules, are opened. These chemicals are extremely important in the process of maintaining the correct proportion of bone production to bone resorption. This increases bone fragility, which is associated with a loss of the ability to detect microdamage and send healing signals.
Pathologic diseases such as osteoporosis and osteoarthritis can be associated with the death of osteocyte cell lines, which can contribute to increased skeletal fragility. Oxygen deprivation, which can occur as a result of inactivity (bed rest), treatment with glucocorticoids, or withholding of oxygen have all been demonstrated to induce osteocyte death. Commonly, osteocytes react to the presence of implant biomaterials in several different ways.
FAQ
What is an osteocyte?
In bone tissue, the form of the mature bone cells is known as an osteocyte, which has dendritic processes and an oblate shape. It is the most common type of cell discovered. Osteocytes can exist for as long as the organism that they are a part of.
Where are osteocytes found?
They are present in the lacunae.
Answer the quiz below to check what you have learned so far about osteocytes.
References
- Dudley, H. R., & Spiro, D. (1961). “The fine structure of bone cells”. The Journal of Biophysical and Biochemical Cytology 11: 627–649.
- Haridy, Y., Osenberg, M., Hilger, A., Manke, I., Davesne, D., & Witzmann, F. (2021). Bone metabolism and evolutionary origin of osteocytes: Novel application of FIB-SEM tomography. Science Advances, 7(14). https://doi.org/10.1126/sciadv.abb9113
- S. National Institutes of Health, N. C. I. (2022). “Structure of Bone Tissue.” Retrieved 23 July, 2022, from https://training.seer.cancer.gov/anatomy/skeletal/tissue.html#:~:text=Between%20the%20rings%20of%20matrix,passageways%20through%20the%20hard%20matrix.
- Tanaka-Kamioka, K., Kamioka, H., Ris, H., & Lim, S. S. (1998). “Osteocyte shape is dependant on actin filaments and osteocyte processes are unique actin-rich projections”. J. Bone Miner. Res. 13 (10): 1555–68.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.