Oxidizer
n., plural: oxidizers
[ˈɒksɪˌdaɪzə]
Definition: Any substance that has the ability to oxidize other substance
Table of Contents
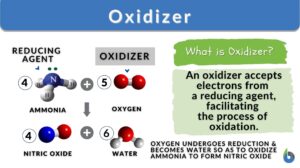
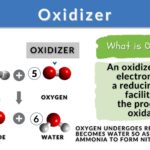
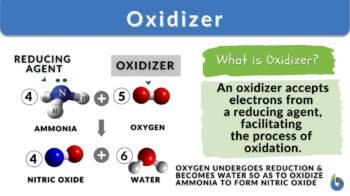
An oxidizer is a substance that facilitates oxidation in a chemical reaction by accepting electrons from another substance. Oxygen is a common oxidizer used in combustion reactions whereas hydrogen peroxide and potassium permanganate are used in everyday life and organic chemistry reactions. Improper handling of oxidizers can lead to dangerous accidents, so proper safety protocols are essential.
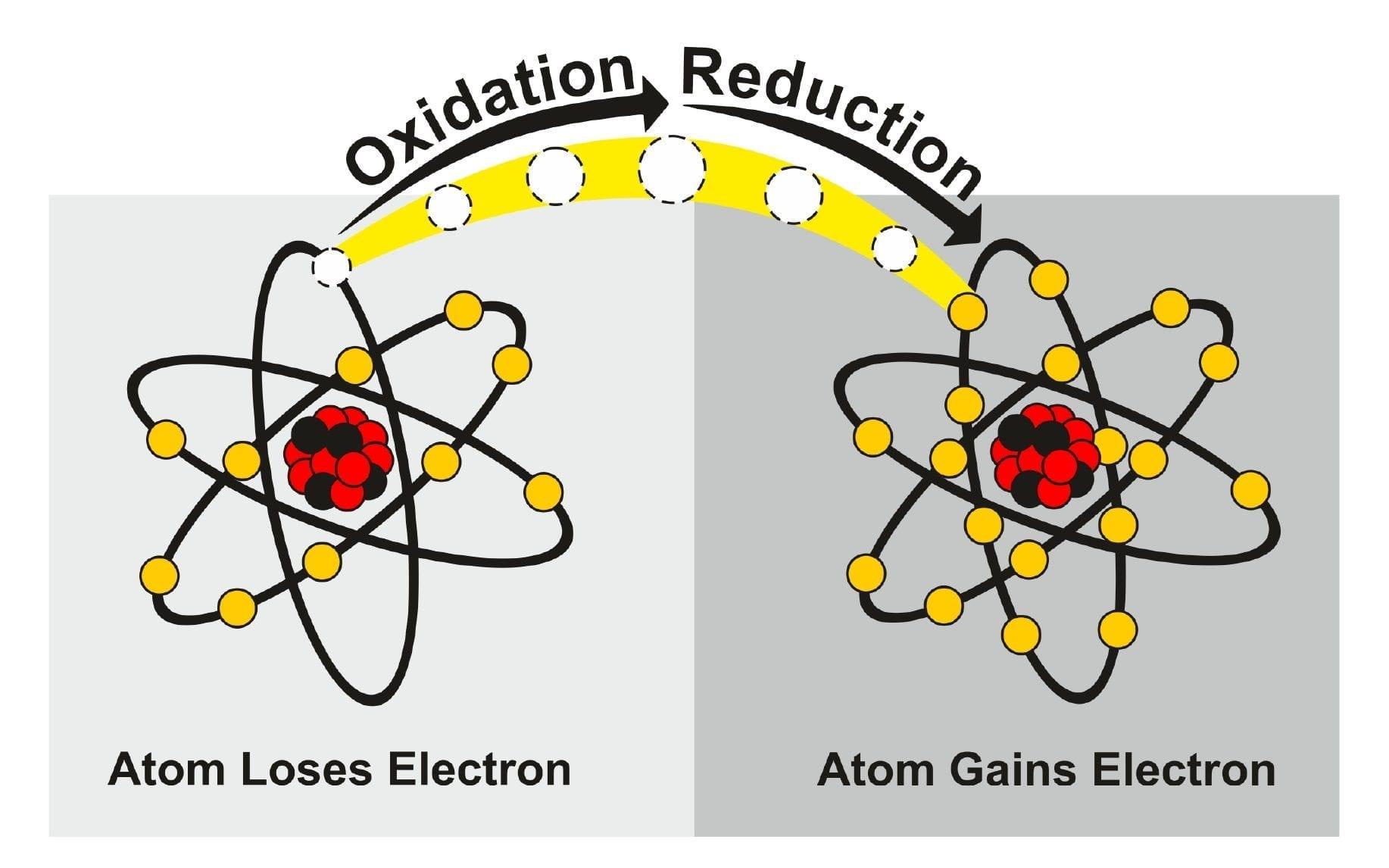
We all have come across the subject of redox reactions in our chemistry or biochemistry classes. Redox reactions refer to chemical reactions that involve the transfer of electrons between two chemical species.
These reactions occur when one species undergoes oxidation by losing electrons while the other undergoes reduction by gaining electrons. The “exchange of electrons” drives the reaction and results in the formation of new chemical compounds.
As we all know, redox reactions are ubiquitous in everyday processes, such as the rusting of iron and the complete combustion of fuels like gasoline in the combustion chamber, as well as in biological processes like cellular respiration and photosynthesis. This makes understanding redox reactions a crucial aspect of various scientific fields such as chemistry, biology, and environmental science. Their practical applications in electricity production through batteries and fuel cells (auxiliary fuel) can’t be overemphasized.
In the subject of redox biology, oxidizer and reducer are prime topics. This article will talk in detail about the former with examples and diagrams. Read on to learn why oxidizers are so important.
What Is an Oxidizer?
An oxidizer, also known as an oxidizing agent, is defined as a substance that facilitates the process of oxidation in a chemical redox reaction by accepting electrons from another substance, which results in the reduction of the oxidizer. In other words, an oxidizer is a compound that causes the oxidation of another compound by taking away its electrons. Because it accepts electrons it is also called an electron acceptor.
Variant: oxidiser (British)
Synonyms: oxidant; oxidizing agent;
See also: redox reaction
Watch this vid to understand the idea of oxidants and reductants.
Importance: Oxidizers are essential in many chemical reactions and are commonly used in industry, research, and everyday life.
Examples:
- Hydrogen peroxide is a common oxidizer used in disinfectants and cleaning products.
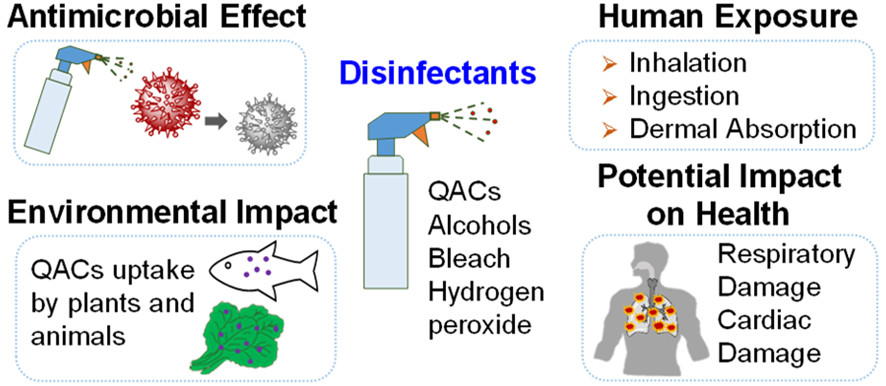
- Potassium permanganate is used as an oxidizer for organic materials.
- One of the most well-known oxidizers is oxygen, which is an essential component of combustion. In combustion reactions, the oxidizer is typically atmospheric oxygen, which reacts with a fuel such as wood or gasoline to produce heat and light.
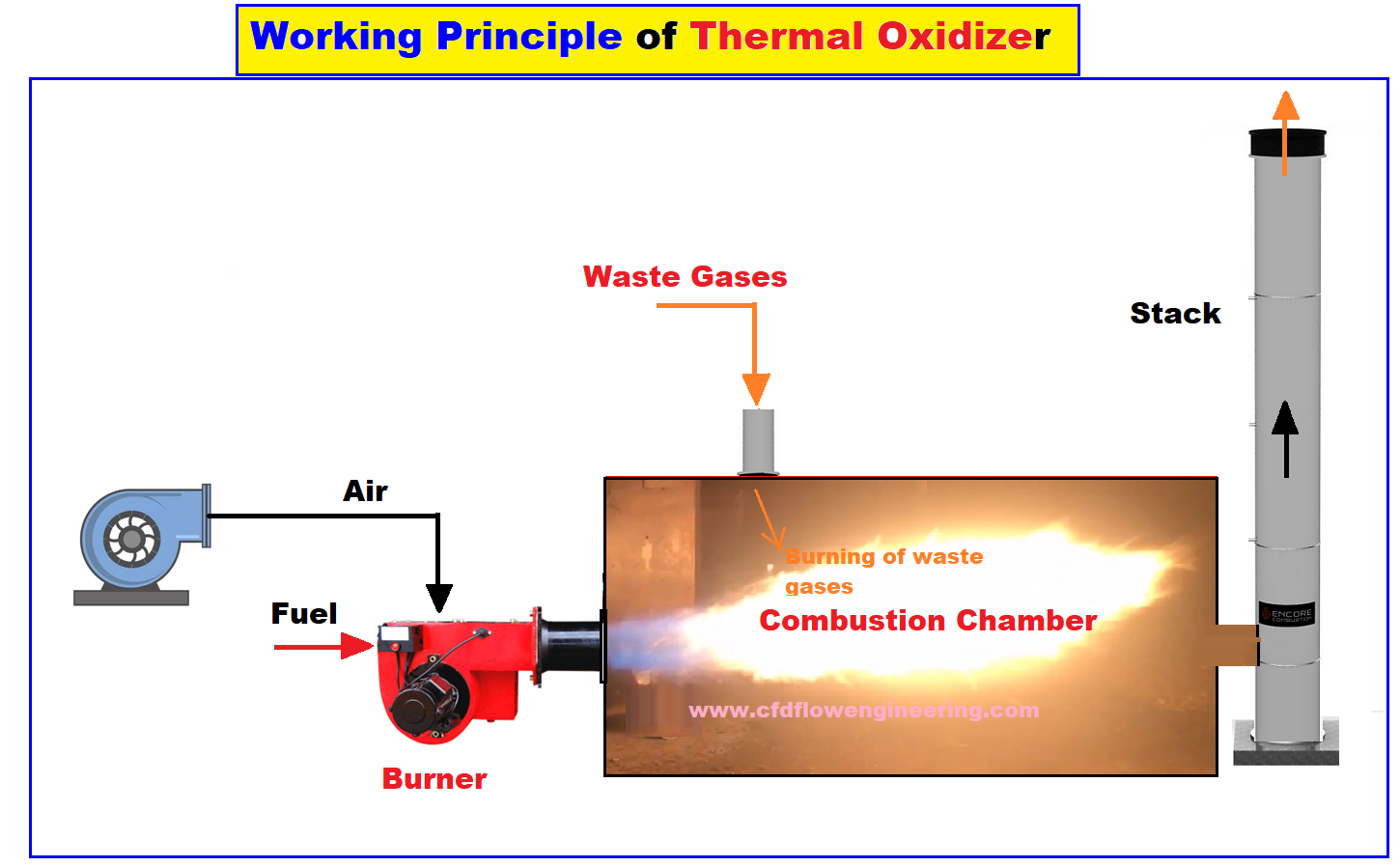
- In waste gas oxidizers, waste gases containing volatile organic compounds (VOCs), organic hazardous air pollutants (HAPs), or odors are combusted in the gas stream to carbon dioxide and water vapor. For this purpose, thermal oxidizers are used. High temperatures are used to combust waste gases containing volatile organic compounds (VOCs), organic hazardous air pollutants (HAPs), or odors into carbon dioxide and water vapor.
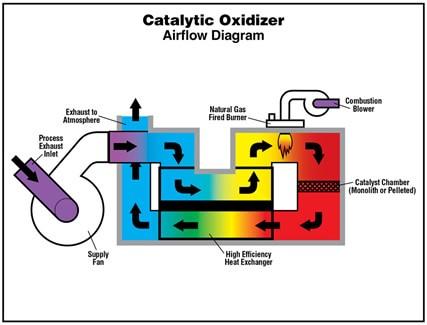
- Catalytic oxidizers use a “catalyst” to facilitate the oxidation process, typically in the form of a honeycomb structure coated with a metal such as platinum, palladium, or rhodium. This catalyst lowers the temperature required for oxidation to occur, reducing the energy needed to operate the system.
- Proper handling and control of SO2 emissions are crucial to prevent environmental and health hazards. The use of a sulfur dioxide oxidizer can help mitigate the negative effects of SO2 emissions by converting them into less harmful compounds.
Warning note: While oxidizers are useful in many applications, they can also be dangerous if not handled properly. For example, certain oxidizers can react explosively with other substances, and the improper storage or handling of oxidizers can lead to serious accidents. Therefore, it is important to handle and store oxidizers by proper safety protocols.

Watch this vid about oxidizers:
Electron Acceptors
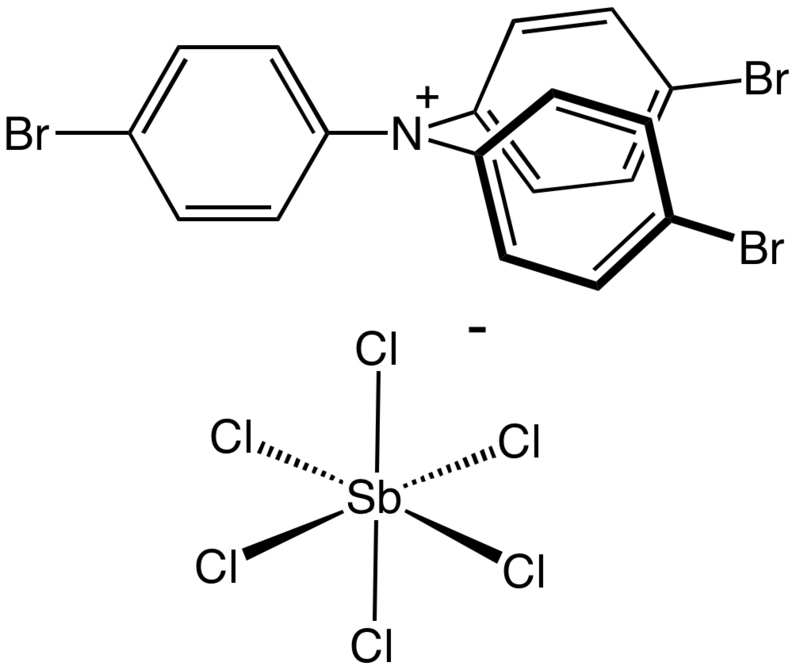
Oxidizers participate in electron transfer reactions. They accept electrons and thus are commonly referred to as electron acceptors. The species from which they take the electrons are called reducing agents/electron donors. There is a variety of electron acceptors in the market of which Magic Blue is the strongest. It is a radical cation that is a derivative of N(C6H4-4-Br)3.
Atom-transfer Reagents
Oxidizers are also commonly seen to transfer oxygen atoms to a substrate. This is the reason they are also referred to as atom-transfer reagents. And this process is known as oxygenation or oxygen-atom transfer reaction (OAT). Some common examples of oxidizers that carry out atom-transfer reactions are:
- MnO−4 (permanganate)
- CrO2−4 (chromate)
- OsO4 (osmium tetroxide)
- ClO−4 (perchlorate)
Note: It is worth noting that all these oxidizing agents are oxides.
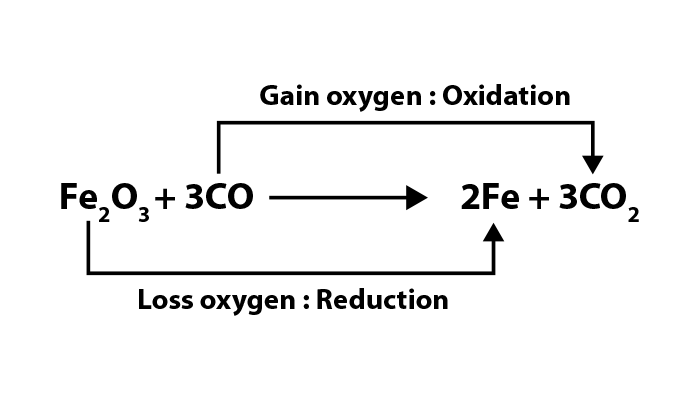
Common oxidizing agents
Several oxidizing agents are important for the efficient functioning of different industries and systems. We list some of the most important oxidizing agents with their roles.
Table 1: Examples of oxidizers with their applications and roles. | |
|---|---|
| Oxidizing Agent | Roles |
| Oxygen | Essential for respiration in all living organisms |
| Ozone | Used for disinfection and sterilization in food processing plants |
| Hydrogen peroxide | Used as a disinfectant and sanitizer in the food industry |
| Fenton’s reagent | Used for degradation of organic pollutants in wastewater treatment plants |
| Halogens | Used for water treatment and as a disinfectant in the food industry |
| Nitric acid and nitrates | Used as a preservative and color fixative in cured meats |
| Potassium chlorate | Used as an oxidizing agent in the production of match heads |
| Sulfuric acid | Used in the production of high fructose corn syrup |
| Peroxydisulfuric acid | Used for sterilization of food contact surfaces and equipment |
| Peroxymonosulfuric acid | Used for disinfection and sterilization in food processing plants |
| Hypochlorite, perchlorate, household bleach, chlorite, chlorate | Used as a disinfectant and sanitizer in the food industry |
| Chromium compounds | Used as a food colorant and preservative |
| Permanganate compounds | Used as a disinfectant and sanitizer in the food industry |
| Sodium perborate | Used as a bleaching agent in food processing |
| Nitrous oxide, Nitrogen dioxide/Dinitrogen tetroxide | Used as a propellant in whipped cream dispensers |
| Sodium bismuthate | Used as an oxidizing agent in the production of acetic acid |
| Cerium (IV) compounds | Used for decolorization and removal of impurities in sugar refining |
| Lead dioxide | Used in the production of food packaging materials |
Data source: Dr. Harpreet Narang of Biology Online.
Dangerous Materials Definition
Oxidizing agents are substances that can cause or contribute to the combustion of other materials. However, not all materials that are classified as oxidizing agents by analytical chemists ‘pass the dangerous goods test’ of an oxidizing agent. For instance, potassium dichromate does not pass the test.
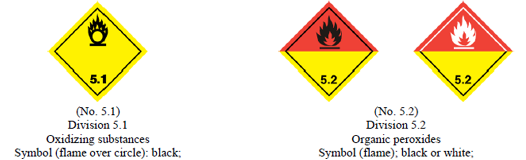
The U.S. Department of Transportation defines oxidizing agents in two categories:
- Class 5; Division 5.1(a)1: Division 5.1 refers to a material that may cause or enhance the combustion of other materials. Division 5.(a)1 applies to solid oxidizers if their mean burning time is less than/equal to the burning time of a 3:7 constitution of potassium bromate and cellulose mixture.
- Class 5; Division 5.1(a)2: This applies to liquid oxidizers if they spontaneously ignite or their mean time for a pressure rise from 690 to 2070 kPa gauge is lower than that of a 1:1 constitution of nitric acid (65%) and cellulose mixture.
Common Oxidizing Agents and Their Products
Some examples of oxidizing agents or oxidizers are detailed below. Their use and products are also described in a little detail.
- Magic Blue: Magic Blue is an oxidizer commonly used in the swimming pool industry. It is a copper-based oxidizer that helps to clarify water by removing organic contaminants and algae.
- Chlorine: Chlorine is a powerful oxidizer for disinfection in water treatment plants, swimming pools, and hot tubs. It works by reacting with and destroying harmful bacteria and viruses.
- Hydrogen peroxide: Hydrogen peroxide is a commonly used oxidizer in the medical industry for wound care and disinfection. It can also be used as a cleaning agent and as a bleaching agent for hair and teeth.
- Potassium permanganate: Potassium permanganate is a strong oxidizer commonly used in the chemical industry for oxidizing organic compounds. It can also be used to purify water and as a disinfectant.
- Nitric acid: Nitric acid is a powerful oxidizer used in the production of fertilizers, explosives, and dyes. It is also used in the metal finishing industry for etching and cleaning metals.
- Potassium chlorate: Potassium chlorate is a strong oxidizer used in the manufacture of fireworks, matches, and explosives.
- Sodium hypochlorite: Sodium hypochlorite is a commonly used oxidizer for disinfection in household cleaning products and swimming pool maintenance.
- Peroxyacetic acid: Peroxyacetic acid is a strong oxidizer used in the food industry for disinfection and preservation of food products.
- Ferric chloride: Ferric chloride is an oxidizing agent used in the water treatment industry for the removal of impurities and in the production of printed circuit boards.
- Ozone: Ozone is a powerful oxidizer used for water and air purification, as well as in the food industry for the disinfection of food products.
- Bromine: Bromine is a strong oxidizer used in the manufacture of flame retardants, pharmaceuticals, and agricultural oxidizing chemicals.
- Potassium persulfate: Potassium persulfate is a strong oxidizer used in the production of polymers, as well as in hair and skin products for bleaching and removing stains.
- Nitrogen dioxide: Nitrogen dioxide is a powerful oxidizing agent used in the chemical industry for the production of nitric acid and as a bleaching agent for wood pulp and textiles.
- Calcium hypochlorite: Calcium hypochlorite is a commonly used oxidizer for disinfection and water treatment, as well as in the manufacture of bleaching agents and chlorinated solvents.
- Sodium chlorite: Sodium chlorite is an oxidizing agent used in the production of chlorine dioxide, a powerful disinfectant and bleaching agent.
- Potassium iodate: Potassium iodate is a strong oxidizer used in the food industry as a dough conditioner and in the production of iodized salt.
- Manganese dioxide: Manganese dioxide is an oxidizing agent used in the production of batteries and as a catalyst in organic synthesis.
- Ammonium persulfate: Ammonium persulfate is a strong oxidizer used in the production of printed circuit boards, as well as in hair bleaching and other cosmetic applications.
- Sodium chlorate: Sodium chlorate is a powerful oxidizer used in the production of bleach and the paper and pulp industry for delignification.
- Lead dioxide: Lead dioxide is an oxidizing agent used in the manufacture of batteries and as a catalyst in chemical reactions.
NOTE IT!
“Oxidizers in Food Industry”
Did you know that oxidizers are not only useful in the water treatment industry but also in the food industry?
Yes, that’s right! Oxidizers such as hydrogen peroxide and peroxyacetic acid are widely used in the food industry for disinfection and preservation of food products. These oxidizers help to kill harmful bacteria and viruses that can cause foodborne illnesses, making food safer for consumption.
But that’s not all! Potassium iodate, another powerful oxidizer, is used as a dough conditioner in the food industry, making bread and other baked goods fluffier and more voluminous. And let’s not forget about the use of ozone, a strong oxidizer, for the disinfection of food products. Ozone can destroy bacteria, viruses, and other harmful microorganisms on the surface of fruits, vegetables, and meat products.
Also, potassium permanganate is used in the food industry as a disinfectant and bleaching agent. It can be used to remove unpleasant tastes and odors from food products such as fish, shrimp, and vegetables. Furthermore, it is used to disinfect water used in food processing and to prevent spoilage of food products during storage and transportation.
Overall, the use of oxidizers in the food industry helps to ensure the safety and quality of our food. From disinfecting food products to improve the texture of baked goods, oxidizers play an important role in our daily lives, even in ways we might not expect, i.e., in our food!!!! 😀

Take the Oxidizers – Biology Quiz!
References
- Dewey, H. M., Jones, J. M., Keating, M. R., & Budhathoki-Uprety, J. (2021). Increased use of disinfectants during the COVID-19 pandemic and its potential impacts on health and safety. ACS Chemical Health & Safety, 29(1), 27-38.
- Hao, X., Li, R., Wang, J., & Yang, X. (2018). Numerical simulation of a regenerative thermal oxidizer for volatile organic compounds treatment. Environmental Engineering Research, 23(4), 397-405.
- Spivey, J. J. (1987). Complete catalytic oxidation of volatile organics. Industrial & Engineering Chemistry Research, 26(11), 2165-2180.
- Dunn, J. P., Koppula, P. R., Stenger, H. G., & Wachs, I. E. (1998). Oxidation of sulfur dioxide to sulfur trioxide over supported vanadia catalysts. Applied Catalysis B: Environmental, 19(2), 103-117.
- Hofmann, A. I., Kroon, R., Zokaei, S., Järsvall, E., Malacrida, C., Ludwigs, S., … & Müller, C. (2020). Chemical doping of conjugated polymers with the strong oxidant magic blue. Advanced Electronic Materials, 6(8), 2000249.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.