
Phenol coefficient
n.,
[ˈfinəl ˌkəʊ.ɪˈfɪʃn̩t]
Definition: a measure of the disinfecting power of a substance
Table of Contents
Chemical disinfectants are categorized based on the power of their disinfection for microbes and viruses. Strong disinfectants can kill fungi, vegetative cells, endospores, and pathogens. Extended use of disinfectants can result in sterilization. Intermediate-level disinfectants are less effective against certain viruses and endospores. Low-level disinfectants are ineffective against endospores and can kill some enveloped viruses and vegetative cells. The measure of the power of disinfection is required to appropriately suggest the application of a particular disinfectant. The effectiveness of chemical disinfectants can be measured using different methods, such as qualitative suspension test, quantitative suspension test, American Association of Official Analytical Chemists (AOAC) Use Dilution test, Kelsey Sykes test, surface time kill test, etc. The phenol coefficient is one of the methods to determine the effectiveness of a disinfectant.
Definition of Phenol Coefficient
The phenol coefficient can be defined as:
“The number obtained by dividing the degree of dilution of test disinfectant by the degree of dilution of phenol in a certain span of time.”
If the number is greater than 1, it means that for given dilution, the test disinfectant is more powerful and can kill germs better than phenol. If the number obtained is less than 1, it means that for the given dilution, phenol is better at controlling germs.
What is Phenol Coefficient?
Phenol is an aromatic compound and a carboxylic acid whose formula is C6H6O. Its structure is shown in the figure below:
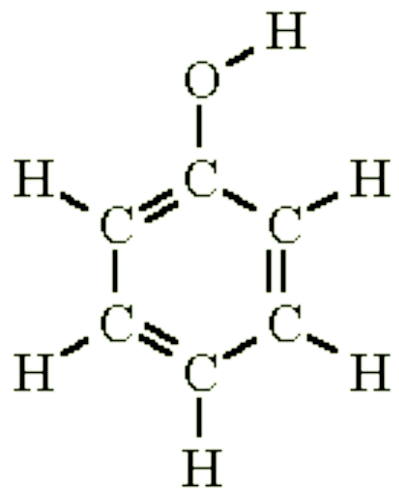
Phenol is recognized as one of the oldest antiseptic agents with excellent antifungal and antibacterial properties. At concentrations of 0.1 % to 1 %, it is bacteriostatic (i.e. stops the reproduction of bacteria). At higher concentrations (from 1% to 2%), phenol is fungicidal and bactericidal (i.e. destroys the fungus or bacteria). Phenol can kill Anthrax spores (which cause severe skin lesions, lung infections, and intestine diseases) at 5% concentration within 48 hours.
Although phenol has excellent antiseptic properties, it is not used as a common antiseptic due to its systemic toxicity on the skin. Death can result from oral ingestion in significant quantities. Thus phenol is used for comparison of the power of disinfection of other disinfectants such as chlorine, ozone, hydrogen peroxide, etc.
Phenol coefficient is a number obtained by dividing dilution ratio test disinfectant with the dilution ratio of phenol under predetermined conditions.
For instance, suppose phenol diluted to 1 part in 100 parts of diluent (1/100) is able to kill an organism in 10 minutes. Another disinfectant is diluted 1 part in 500 parts diluent (1/500) is able to kill organisms at the same time. So the phenol coefficient can be calculated as follows:

Thus, the phenol coefficient is 5. It means that the test disinfectant is stronger than phenol in terms of disinfection.
Phenol Coefficient Test
Two types of phenol coefficient tests are done:
- Rideal Walker method for phenol coefficient determination
- Chick Martin test
Rideal Walker Method for Phenol coefficient determination
In 1903, Rideal Walker proposed a method to determine the power of a disinfectant in comparison with phenol. Let’s take a sample experimental procedure as follows.
- The broth with a test organism (Salmonella typhi) is prepared using microbiological grade meat extract (20g), sodium chloride (10g), microbiological grade peptone (20g), and distilled water 1000ml.
- The solids are mixed in water and boiled, sterilized, and then brought to room temperature.
- The pH of the solution is maintained between 7.3 and 7.5 using hydrochloric acid. The test organism Salmonella typhi culture is maintained by weekly sub-culture on nutrients. The sub-culture is added to the broth.
- The phenol solutions are prepared by dissolving 1 gram phenol in i.e. 95, 100, 105, 155 ml of water.
- Similarly, the desired concentrations of test disinfectant are prepared.
- Subcultures are tested on plates for intervals of 2.5, 5, 7.5, and 10 minutes.
The following table shows sample empirical data:
| Disinfectant | Dilution ratio | Time | |||
|---|---|---|---|---|---|
| 2.5 | 5.0 | 7.5 | 10 | ||
| Phenol | 1:95 | + | – | – | – |
| 1:100 | + | + | – | – | |
| 1:105 | + | + | + | – | |
| 1:155 | + | + | + | + | |
| Test disinfectant | 1:100 | + | – | – | – |
| 1:200 | + | – | – | – | |
| 1:250 | + | + | – | – | |
| 1:300 | + | + | + | + | |
| 1:350 | + | + | + | + | |
| 1:400 | + | + | + | + | |
The phenol coefficient for the above sample can be calculated as:

The limitation of the Rideal Walker method is that it does not account for the presence of any organic matter. Moreover, the time for disinfection testing is too short. This test is used only to determine the power of phenolic type disinfectants only.
Chick Martin test
Chick martin test incorporates the presence of organic matter as the test is not carried out in the water but yeast suspension or 4% dried human feces. The total time of the test is 30 minutes. Both S. typhi and S. aureus cultures are used to test the efficacy of disinfectants. The calculation method is the same as that Rideal Walker test.
Limitations of Phenol Coefficient Tests
The phenol coefficient tests are designed specifically for determining the disinfection power of phenol-like disinfectants. However, it has been observed that the phenol coefficient is used for other purposes for which it is not applicable. Some chemicals whose structure and properties are completely different from phenol but are germicides (such as chlorine, picric acid, hydrogen peroxide, formalin, iodine, etc.) have been compared with phenol. In some cases, water-insoluble compounds are compared with phenol either in their pure form or diluted in other kinds of solvents. Such misuse of phenol coefficient creates confusion. It is also not recommended to use phenol coefficient for testing antiseptics because antiseptics are not used to kill Bacillus typhosus usually used in phenol tests. Different antiseptics kill different bacteria with varying antiseptic power. One example is tincture iodine, which is 760 times more disinfecting compared to 5 % phenol solution. However, in reality, tincture iodine is not 760 times more germicidal than 5 % phenol in practical conditions (Reddish, 1937).
Factors Affecting Phenol Coefficient Test
Four major factors affect the disinfectants and thus produce wrong results for phenol coefficient tests. These factors are temperature, pH, surface activity, and the presence of interfering substances. An increase in temperature has shown increased disinfectant properties. Optimal growth is achieved at pH between 6 to 8; thus, the recommended pH for the tests is 7.5. The surface-active compounds in low concentrations may increase the disinfectant power. Interfering substances such as certain salts may hinder disinfectant activity (Ononugbo, Reward, & Ike, 2018).
Try to answer the quiz below and see what you have learned so far about phenol coefficient.
References
- Chem.Purdue. (2019). Phenol. Retrieved June 21, 2021, from https://www.chem.purdue.edu/jmol/molecules/phenol.html
- Lakomia, L. & Fong, E. (1999). Microbiology for health careers. Albany, NY: Delmar Publishers.
- Ononugbo, C., Reward, E., & Ike, A. (2018). The Effect of pH and Temperature on Phenol Coefficients of Two Common Disinfectants Using Clinical Isolates of Escherichia coli and
- Staphylococcus aureus. Journal of Advances in Microbiology, 10(2), 1–7. https://doi.org/10.9734/jamb/2018/41376
- Reddish, G. F. (1937). Limitations of the Phenol Coefficient. Industrial and Engineering Chemistry, 29(9), 1044–1047. https://doi.org/10.1021/ie50333a017
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.








