Phosphate
n., plural: phosphates
[ˈfɑsfeɪt]
Definition: A biological molecule consisting of phosphorus and oxygen
Table of Contents
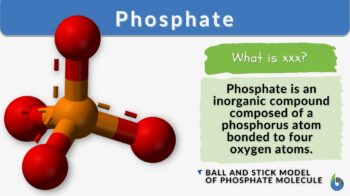
Phosphate is an essential inorganic compound composed of a phosphorus atom bonded to four oxygen atoms. This molecular structure is a fundamental building block for many vital biological processes, including cellular signaling, DNA and RNA synthesis, and energy production. Beyond its biological importance, phosphates are widely used in various industrial applications, such as fertilizers and detergents.
Biology can be conveniently described as “chemistry in action”. Living organisms are essentially complex chemical systems, with interactions between biological molecules driving everything from metabolism to gene expression. Understanding these chemical entities and interactions is essential to understanding how living organisms work.
Of all the chemical entities essential for biological function, phosphate is a vital one with varied roles in Biology ranging from energy metabolism, nucleic acids, and enzyme regulation to membrane structure. It’s a superstar that powers many biological processes and is essential to life.
In this article, we will comprehensively deal with phosphate, its biological significance, and its chemical properties while focusing on many other aspects related to plants, human health, and industrial production.
What Is Phosphate
The chemical definition of phosphate encompasses its polyatomic, anionic, and ester-like nature. A phosphate is defined as an ester of phosphoric acid (H3PO4). It can also be called a “derivative” of phosphoric acid. When generally referring to phosphoric acid, researchers usually mean orthophosphoric acid which is denoted by H3PO4.
Phosphate is a vital nutrient for plants and animals, but it’s important to note that only a fraction of the total phosphorus found in nature is in a form that can be easily used. This fraction must be carefully managed to ensure sustainable use and prevent environmental harm.
If you wish to learn more about the different types of phosphoric acids, an interesting read awaits you at the end of this article (jump to Factoid!) ☺
Phosphate is an essential inorganic compound composed of a phosphorus atom bonded to four oxygen atoms. As a biological molecule, it plays a major role in the biological processes of many organisms, such as a chemical component of nucleic acids (DNA and RNA), nucleotides (ATP), plasma membrane (phospholipids), etc.
Formula: PO₄³⁻
Chemical Properties
» Symbol to denote a phosphate ion = [PO4]3−
» Since the ion is derived from orthophosphoric acid, it is also called an orthophosphate ion.
» Number of protons (H+) removed for the formation of phosphate ion = 3
» Variations of phosphate ions (in terms of loss of protons from phosphoric acid, i.e. H3PO4):
- Dihydrogen phosphate ion [H2PO4]− , formed by removal of 1 proton from H3PO4
- Hydrogen phosphate ion [HPO4]2−, formed by removal of 2 protons from H3PO4
- Phosphate ion [PO4]2−, formed by removal of 3 protons from H3PO4
» Variations of phosphate ions (in terms of the addition of protons to phosphate ions, i.e. [PO4]2−):
- Dihydrogen phosphate ion [H2PO4]−, formed by the addition of 2 protons to phosphate ion [PO4]2− or the addition of 1 proton to hydrogen phosphate ion [HPO4]2−
- Hydrogen phosphate ion [HPO4]2−, formed by the addition of 1 proton to phosphate ion [PO4]2−
» Roles in Biological and Chemical processes: dihydrogen phosphate, hydrogen phosphate, and phosphate are the three forms of the phosphate ion with important functions in biological systems.
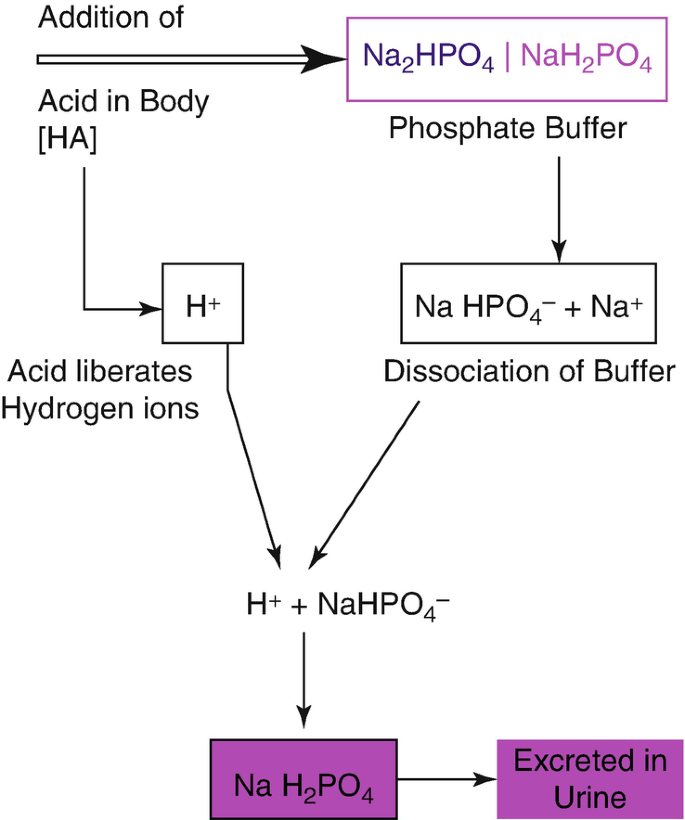
- Dihydrogen phosphate: An important buffer in biological systems, helping to regulate pH in cells and maintaining the acid-base balance in the blood.
- Hydrogen phosphate: Important for bone health and teeth mineralization and also plays a role in regulating pH in biological systems.
» Phosphate: An important component of DNA, RNA, and ATP (adenosine triphosphate), which is the primary energy source for many cellular processes.

» While terms like phosphate, dihydrogen phosphate, and hydrogen phosphate are discussed here as ionic entities, these terminologies are also used to describe the salts of these ions (to be specific, negatively-charged ions or anions).
» When combined with different cations, the different phosphate ions form salts of phosphates, which have a variety of uses and applications. The properties and uses of these salts of phosphates depend on their specific composition and the intended application. Salts of phosphates are used in a wide variety of fields, such as food and beverage production, agriculture, medicine, and industry. Some examples of the salts of phosphates are:
- Phosphate salts:
Trisodium phosphate
Calcium phosphate
Ammonium phosphate - Hydrogen phosphate salts:
Disodium phosphate
Tricalcium phosphate
Sodium hexametaphosphate - Dihydrogen phosphate salts:
Monoammonium phosphate
Monosodium phosphate
Dicalcium phosphate dehydrate
» The molar mass of phosphate ion = 94.97 g/mol
» Molecular structure = A central phosphorus atom (denoted by P) and 4 oxygen atoms (denoted by O) surrounding it in a tetrahedral type of arrangement.
» Terminologies concerning conjugate base concept:
- Phosphate ion = Conjugate base of H(PO4)2− (hydrogen phosphate ion)
- Hydrogen phosphate ion = Conjugate base of H2(PO4)− (dihydrogen phosphate ion)
- Dihydrogen phosphate ion = Conjugate base of H3PO4 (orthophosphoric acid).
» Water solubility = Mostly soluble at standard temperature and pressure conditions
Examples of some water-soluble salts of phosphate:
Sodium phosphate
Potassium phosphate
Rubidium phosphate
Cesium phosphate
Ammonium phosphate
Examples of partially soluble or insoluble salts of phosphate:
Calcium phosphate (sparingly soluble)
Iron (III) phosphate (sparingly soluble)
Aluminum phosphate (insoluble)
Lead (II) phosphate (insoluble)
Higher solubility of hydrogen and dihydrogen phosphates: In general, the solubility of hydrogen and dihydrogen phosphates is slightly higher than that of the corresponding phosphates. This can be attributed to the presence of additional hydrogen ions in hydrogen and dihydrogen phosphates, which increase the solubility of the salts by “enhancing the ion-dipole interactions” with water molecules.
Equilibria in solution
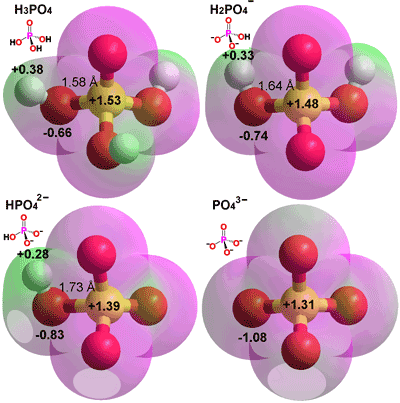
Phosphate ions are found in water and can exist in different forms depending on how acidic or basic the water is. At a neutral pH, one type of phosphate ion is more common, while at lower or higher pH, different types of phosphate ions become more common. These different types of phosphate ions can be represented by different chemical reactions.
» Dominant species at different pH conditions:
At a pH lower than 1, the H3PO4 is the dominant species.
At lower pH (below 7), the H₂PO₄⁻ ion becomes more prevalent.
At neutral pH (around 7), the HPO₄²⁻ ion becomes more prevalent.
At higher pH (above 13), the PO₄³⁻ ion becomes more prevalent.
» The equilibrium of phosphate in solution can be represented by the following reactions:
H3PO4 ⇌ H₂PO₄⁻ + H⁺
H₂PO₄⁻ ⇌ H⁺ + HPO₄²⁻
HPO₄²⁻ ⇌ H⁺ + PO₄³⁻
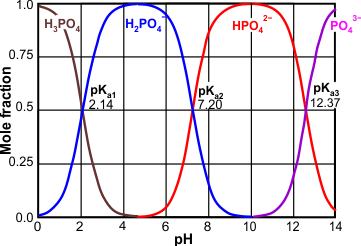
» The equilibrium constants for these reactions can be calculated using the dissociation constants (pKa values) of the various forms of phosphate.
» The exact concentrations of these species at equilibrium will depend on factors such as the pH, temperature, and the concentrations of other ions present in the solution.
» Phosphate ions can also attach to other ions like metals, which can affect how easy it is for plants and animals to use them.
Biochemistry of phosphates
Phosphorus is an important element in biological systems and can be found in two major forms: inorganic phosphates and organic phosphates.
- Inorganic phosphate is denoted as Pi and is present as free phosphate anions in solution. It can exist in various forms depending on the pH of the solution. At physiological pH, inorganic phosphate consists primarily of a mixture [HPO4]2− and [H2PO4] − ions. The concentrations of these ions in the cytosol depend on the pH of the solution. Pyrophosphate anions are also a type of inorganic phosphate.
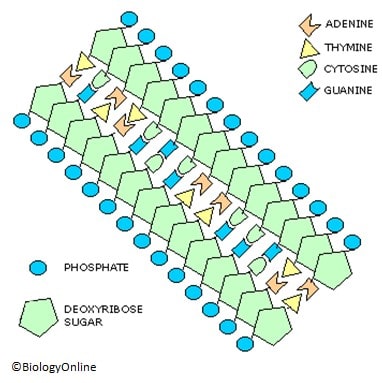
- Organic phosphates are generally found in the form of esters. Some examples of esters are nucleotides and DNA/RNA. In these molecules, phosphorus is bound to other atoms, including oxygen, carbon, and nitrogen. The hydrolysis of the phosphoanhydride bonds leads to the release of free orthophosphate anions and a large amount of potential energy. This usually happens to ATP or ADP (both being high-energy phosphates), thus appropriately making ATP the “energy currency of the cell”.
The readily available source of energy for many metabolic processes:
- Phosphorylation reaction
- Dephosphorylation reaction
These reactions involve the transfer of phosphate groups from ATP to other molecules like proteins and sugars. These reactions are usually catalyzed by enzymes and regulated by various factors.
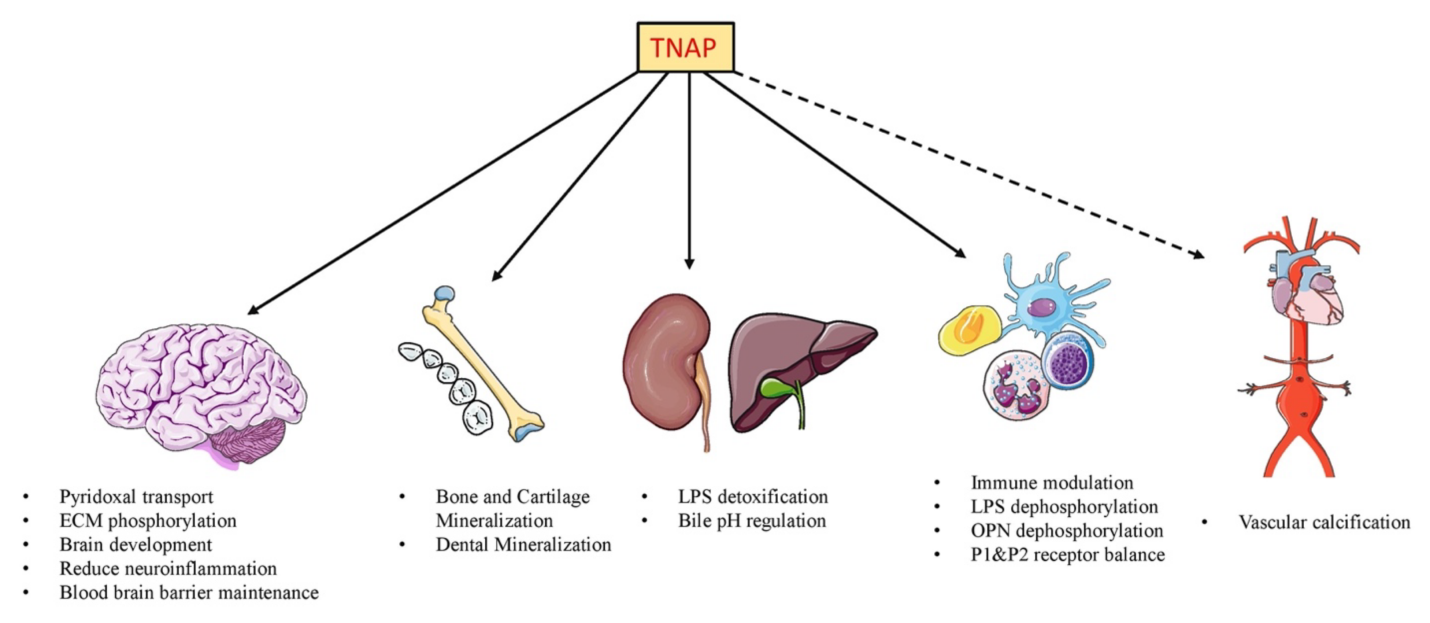
Nonspecific alkaline phosphatase is an enzyme that can be found in various tissues of the body and is involved in numerous physiological processes, including bone mineralization and liver function.
Phosphate is an important nutrient for plants and is often a limiting factor in plant growth. In aquatic environments, excessive phosphate levels can cause eutrophication and harm aquatic life.
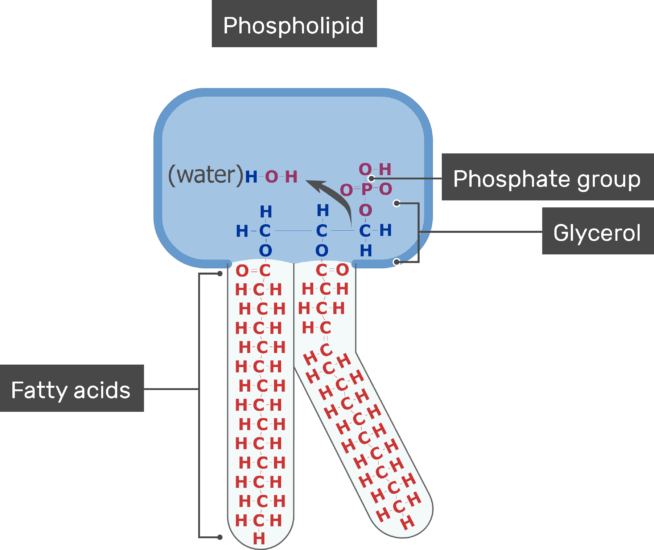
Phosphate is a crucial component of cell membranes, providing structural support and allowing for important cellular processes like signaling and transport.
Matrix vesicles are small extracellular vesicles that play a crucial role in the mineralization of bone and cartilage by providing a site for the nucleation and growth of hydroxyapatite crystals, which are composed of calcium and phosphate.
Bones and teeth
Phosphate plays a critical role in the formation and maintenance of bones and teeth. It combines with calcium to form hydroxyapatite, the mineral that gives bones and teeth their strength and rigidity. Fluoroapatite, a type of hydroxy calcium phosphate in which some hydroxyl groups are “substituted with fluoride ions”, may be present in the dense and sturdy enamel of mammalian teeth. Without adequate phosphate levels, bones and teeth would be weaker and more prone to fractures and decay.

Related Diseases
The role of phosphate in bones and teeth is indispensable. Some diseases that can be related to this essential element include:
- Osteoporosis: A condition of bones weakening occurring often due to a loss of calcium and phosphate.
- Rickets: A condition affecting children and caused due to the lack of vitamin D, which is essential for calcium and phosphate reabsorption in the body. Since less absorption occurs, this eventually leads to weakened bones and stunted growth.
- Hypophosphatemia: A condition characterized by low levels of phosphate in the blood which can lead to weakened bones, muscle weakness, and other complications.
- Hyperphosphatemia: A condition characterized by high levels of phosphate in the blood which can lead to calcification of soft tissues, chronic kidney disease (like renal failure), and other complications.
Medical and biological research uses
Phosphates have many important applications in medical and biological research, and their diverse properties and functions make them an essential component of many biological processes.
Phosphates are important components of many biological molecules, including DNA, RNA, and ATP, and researchers use phosphate-based compounds for the development of new drugs that can target specific enzymes or pathways involved in various diseases.
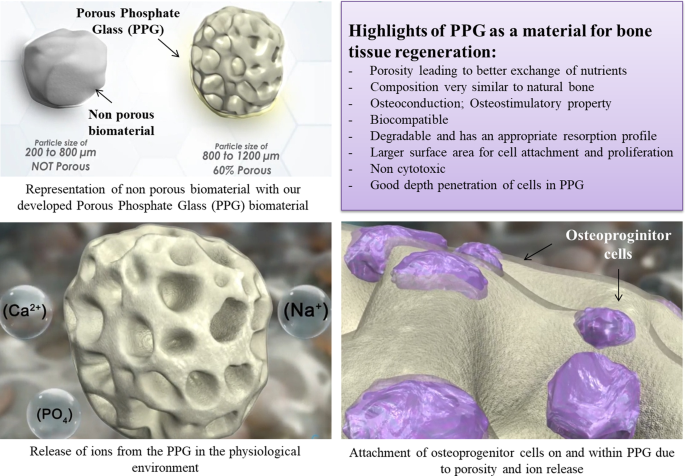
Phosphates are used in the development of biomaterials and tissue engineering scaffolds, such as hydroxyapatite, which is commonly used in bone grafts and dental implants.
Phosphates play a crucial role in cellular signaling pathways, including those involved in cell growth, division, and differentiation and researchers use phosphate-based probes and assays to study these pathways and research how they are regulated.
Phosphates can be used in various diagnostic tests to detect diseases such as osteoporosis, kidney disease, and certain types of cancer.
Phosphates are important components of fertilizers and are used in agricultural research to study plant growth and development.
Phosphates are used to treat urinary tract infections by making the urine more acidic, thus helping to alleviate symptoms.
Certain phosphates can help prevent the formation of calcium stones in the urinary tract.
Phosphate binders are medications used to reduce the absorption of dietary intake phosphate in the digestive system, commonly prescribed to patients with kidney disease.
Phosphates are also used as dietary supplements in cases where patients are unable to obtain sufficient phosphorus from their diet due to certain diseases or disorders. Warning note: However, it’s important to note that injectable phosphates must only be administered by qualified healthcare professionals.
Phosphate is an important ion in the extracellular fluid, where it plays a crucial role in a variety of physiological processes, such as bone mineralization and energy metabolism. However, excessive levels of phosphate in the extracellular fluid can lead to health issues, making the regulation of phosphate levels crucial.
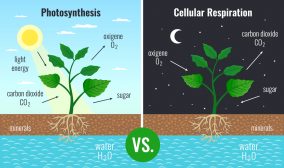
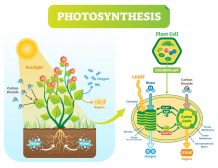
Plant metabolism
Plants absorb phosphorus through multiple pathways, with two of the most significant ones being the arbuscular mycorrhizal (AM) pathway and the direct uptake pathway. Both the AM and direct uptake pathways are essential for plant growth and development. The significance of each pathway depends on various factors, including plant species, soil conditions, and availability of phosphorus.
- The AM pathway is a type of symbiotic relationship between plants and fungi. The fungi form arbuscules on the roots of plants, which help transfer nutrients, including phosphorus, from the soil to the plant. This pathway is prevalent in many types of plants, such as legumes, grasses, and several crops.
- The direct uptake pathway, on the other hand, involves the direct absorption of phosphorus from the soil through the roots of the plants. This pathway is regulated by proteins on the surface of the root cells, which facilitate the transport of phosphate ions into the plant cells. This pathway becomes more important when soil phosphorus levels are high and the efficiency of the mycorrhizal pathway decreases.

Adverse Health Effects
The most notable health effect of excessive concentration of blood serum phosphate (exceeding 4.5 mg/dL) in humans and animals is called hyperphosphatemia. This condition is marked by symptoms like:
- Joint and bone pain
- Muscle weakness and cramps
- Itching and rash
- Nausea and vomiting
- Fatigue and weakness
- Difficulty breathing
Kidney failure is the most common cause of hyperphosphatemia in human beings, cats, and dogs. It is recommended to avoid restricting dietary phosphate by avoiding food with a high phosphate-to-protein ratio like processed foods in such conditions. Hypoparathyroidism is another medical condition that causes hyperphosphatemia. In hypoparathyroidism, the parathyroid gland produces insufficient amounts of parathyroid hormone; the hormone which helps to regulate calcium and phosphate levels in the blood.
Watch this vid about phosphate, its importance, and health conditions, hypophosphatemia, and hyperphosphatemia :
Production
Phosphates are produced in nature through the weathering of rocks and minerals, as well as through the decay of organic matter, which releases phosphorus into the soil.
Geological occurrence
Phosphates are naturally occurring in phosphate rocks, which are mined to obtain phosphorus for use in agriculture and industry. Morocco is the largest global producer and exporter of phosphates, while the Bone Valley region in Florida, the Soda Springs region in Idaho, and the coast of North Carolina contain the largest deposits in North America. Other countries with large phosphate-mining industries include Egypt, Israel, Tunisia, and Australia. Scientists predict that phosphorus reserves will be depleted within the next 50-100 years due to the current rate of consumption, with “peak phosphorus” expected to occur in 30 years.
Mining
Approximately 70% of global phosphate production is attributed to the top three producing nations, namely China, Morocco, and the United States.
Ecology
Phosphate is an important resource in ecological terms due to its role in biological systems, as it often limits growth rates in freshwater environments. Excessive levels of phosphate can lead to ecological consequences such as population blooms, oxygen deprivation, and eutrophication. Phosphate deposits may also contain heavy metals, and mining operations can produce waste products that pose an increased risk to groundwater and marine life. Bacteria and clay minerals in alluvial topsoil can result in calcium hydroxyapatite and calcite precipitates, promoting biomineralization.
NOTE IT!
Varieties of Phosphoric Acids in the Chemical World!!
Phosphoric acid is a versatile and widely used chemical compound that exists in several forms, each with unique properties and applications. Let’s dive into the different types of phosphoric acids and what makes them interesting.
- Orthophosphoric acid (H3PO4): This is the most common type of phosphoric acid and is used in a variety of applications, including fertilizer production, food, and beverage processing, and as a rust remover. Orthophosphoric acid is a triprotic acid, meaning it has three acidic hydrogen atoms that can be ionized.
- Pyrophosphoric acid (H4O7P2): This type of phosphoric acid is formed by the dehydration of two molecules of orthophosphoric acid. Pyrophosphoric acid is used in the production of detergents, water treatment, and as a catalyst in organic reactions.
- Tripolyphosphoric acid (H5O10P3): This type of phosphoric acid is formed by the polymerization of orthophosphoric acid molecules. Tripolyphosphoric acid is used in the production of detergents, and ceramics, and as a water softener.
- Polyphosphoric acid: This type of phosphoric acid is a mixture of orthophosphoric acid and other phosphoric acids, including pyrophosphoric acid and tripolyphosphoric acid. Polyphosphoric acid is used as a catalyst in organic reactions, in the production of plastics, and as a stabilizer in the production of synthetic fibers.
Take the Phosphate – Biology Quiz!
Further Reading
References
- Gupta, A. (2019). Acid-Base Balance. In: Comprehensive Biochemistry for Dentistry. Springer, Singapore. https://doi.org/10.1007/978-981-13-1035-5_20
- Sekaran, Saravanan, et al. “The Physiological and Pathological Role of Tissue Nonspecific Alkaline Phosphatase beyond Mineralization.” Biomolecules, vol. 11, no. 11, 2021, p. 1564, https://doi.org/10.3390/biom11111564. Accessed 1 May 2023.
- Dissanayake, Shama, et al. “Identification of Key Functional Motifs of Native Amelogenin Protein for Dental Enamel Remineralisation.” Molecules, vol. 25, no. 18, 2020, p. 4214, https://doi.org/10.3390/molecules25184214. Accessed 1 May 2023.
- Chauhan, N., Lakhkar, N. & Chaudhari, A. Development and physicochemical characterization of novel porous phosphate glass bone graft substitute and in vitro comparison with xenograft. J Mater Sci: Mater Med 32, 60 (2021). https://doi.org/10.1007/s10856-021-06532-8
- Seleiman, M. F. (2014). Towards sustainable intensification of feedstock production with nutrient cycling.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.