Protein
n., ˈpɹəʊtiːn
In biology, a protein is a biomolecule comprised of amino acid residues joined together by peptide bonds.
Table of Contents
Protein Definition
Proteins are biomolecules comprised of amino acid residues joined together by peptide bonds. Biomolecules are molecules produced by living organisms. As such, most of them are organic molecules. Proteins are one of the major biomolecules. The others are carbohydrates (especially, polysaccharides), lipids, and nucleic acids. The components of proteins include carbon, hydrogen, oxygen, sulfur, nitrogen, and sometimes phosphorus. (Ref. 1)
Proteins differ from each other through their amino acid composition and sequence, location, function, and spatial configuration. The amino acids in a protein are determined by the nucleotide sequence of the gene coding for them. The amino acid sequence often determines how the protein folds into a particular 3D configuration. This, in turn, determines the activity and function of the protein.
In biological systems, proteins have varying functions. Some of them are structural materials (e.g. keratin) whereas others act as enzymes. Other functions include transport (e.g. hemoglobin), immunity (e.g. antibodies), and regulation.
In biology and biochemistry, a protein is a biomolecule or a macromolecule characterized by being made up of chain(s) of amino acids joined together by peptide bonds. In nutrition, a protein refers to food rich in biomolecular proteins and provides about 4 cal/gram food energy. (Ref. 2)
Etymology: The term protein came from French protéine, from Late Greek prōteios, of the first quality, from Greek prōtos, meaning “first”.
Protein vs. Peptide
A peptide is a compound consisting of amino acids connected by a peptide bond, particularly between the carboxyl group of one amino acid the amine group of another amino acid. (Ref. 3) When a peptide is comprised of two amino acids, it is called a dipeptide. A polypeptide is a peptide comprised of several amino acid residues (as many as 4,000) forming an unbranched linear chain. (Ref. 4) Many peptides contain fewer than 20 to 30 amino acid residues (Ref. 4); they are referred to as oligopeptides (short polypeptides). A protein is a polypeptide or a complex of polypeptides with a 3D structure and carries out a particular function. (Ref. 4)
Structure of Protein
Proteins are composed of polymers of amino acid residues. The amino acids are joined together by peptide bonds. Each protein is a linear polymer built from different amino acids. The type and the sequence of amino acids in a protein are specified by the DNA in the cell that produces them. The genetic code typically specifies 20 standard amino acids. However, some organisms, such as archaea, have a genetic code that specifies more. This sequence of amino acids is essential since it determines the overall structure and function of a protein. Some proteins can form a complex together with another protein. Others form a complex with other biomolecules other than the peptide. Some of these non-peptide groups in a protein are referred to as cofactors or prosthetic groups. (Ref. 5)
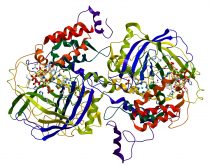
There are four distinct types of protein structure: (1) primary structure, (2) secondary structure, (3) tertiary structure, and (4) quaternary structure. (Ref. 6)
- The primary structure pertains to the sequence of amino acids in a polypeptide chain.
- The secondary structure of a protein refers to the regularly repeating local structures that are stabilized by hydrogen bonds. Examples of a secondary structure are α-helix, β-sheet, and Turns and Loops.
- The tertiary structure (sometimes called fold) pertains to the spatial relationship of the secondary structures from one to another. It is often stabilized by nonlocal interactions, e.g. by disulfide bonds, salt bridges, and the formation of a hydrophobic core. This structure of the protein is what determines the fundamental function of the protein.
- The quaternary structure refers to the protein complex, i.e. when several polypeptide chains (sometimes referred to as protein subunits) constitute it. The tertiary and quaternary structures are often called conformations. The protein may transition from one structure to another and the transition between tertiary and quaternary by a conformational change. Conformational changes may be induced for example when a substrate binds to a protein (particularly, an enzyme) at its active site.
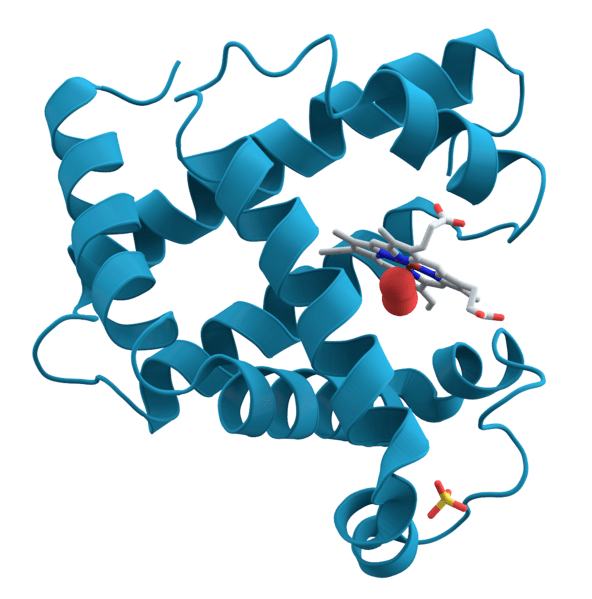
Explore thousands of fascinating biological and chemical structures easily on an iPad: Molecule WorldTM for iPad by Digital World Biology.
Types
A protein may be classified based on its form and main functions: it can be a globular protein like most enzymes, a fibrous protein (with a structural role, such as collagen, keratin, etc.), or a membrane protein that serves as a receptor or a channel (where a polar or a charged molecule can move into the cell across the cell membrane). (Ref. 7)
Biosynthesis of Proteins
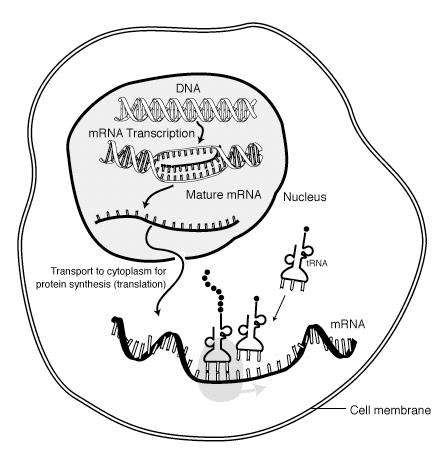
Protein synthesis is the creation of proteins. In biological systems, it is carried out inside the cell. In prokaryotes, it occurs in the cytoplasm. In eukaryotes, it initially occurs in the nucleus to create a transcript (mRNA) of the coding region of the DNA. The transcript leaves the nucleus and reaches the ribosomes for translation into a protein molecule with a specific sequence of amino acids. Protein synthesis (also called protein biosynthesis when performed by a living organism) is a process of creating protein molecules. In biological systems, it involves amino acid synthesis, transcription, and translation. In amino acid synthesis, there is a set of biochemical processes that produce amino acids from carbon sources like glucose. Not all amino acids are produced by the body; other amino acids are obtained from the diet.
READ: Protein Synthesis and Protein Activity and Cellular Metabolism
Degradation
The lifespan of proteins varies depending on their kind. Some proteins are degraded after a few minutes of production; others remain for years. Misfolded proteins are degraded at once to prevent them from causing damage due to their instability and dysfunctional nature. The cell may degrade or recycle them through protein turnover. Proteins may be degraded in proteasomes, i.e. cytoplasmic complexes that digest worn-out or misfolded proteins tagged with ubiquitin. (Ref. 11) In eukaryotes, another site of protein degradation is the lysosome. Lysosomes contain proteases that digest endocytosed proteins.
Biological functions
Proteins have several functions. Some of them are structural components (e.g. keratin in hair, actin, and myosin in muscle, etc.). Many proteins are enzymes. They catalyze various biochemical reactions and therefore are essential to metabolism. Other vital roles of proteins in biological systems are as follows: as transporters (e.g. hemoglobin), as antibodies, and as regulators of gene expression. Not all proteins are biosynthesized de novo. They may be acquired through diet. In fact, animals require a protein diet to obtain the essential amino acids that they cannot synthesize on their own. Thus, in this regard, dietary proteins serve as a food source.
READ: Different Types of Protein – Tutorials
Try to answer the quiz below to check what you have learned so far about proteins.
Further Reading
References and further reading
- Proteins. (2020). Gsu.Edu. http://hyperphysics.phy-astr.gsu.edu/hbase/Organic/protein.html
- How many calories are in one gram of fat, carbohydrate, or protein? | Food and Nutrition Information Center| NAL | USDA. (2016). Usda.Gov. https://www.nal.usda.gov/fnic/how-many-calories-are-one-gram-fat-carbohydrate-or-protein
- Berg, J. M., Tymoczko, J. L., & Lubert Stryer. (2017). Primary Structure: Amino Acids Are Linked by Peptide Bonds to Form Polypeptide Chains. Nih.Gov; W H Freeman. https://www.ncbi.nlm.nih.gov/books/NBK22364/
- Lodish, H., Berk, A., S Lawrence Zipursky, Matsudaira, P., Baltimore, D., & Darnell, J. (2017). Hierarchical Structure of Proteins. Nih.Gov; W. H. Freeman. https://www.ncbi.nlm.nih.gov/books/NBK21581/
- Proteins. (2010). Msu.Edu. https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/protein2.htm
- Berg, J. M., Tymoczko, J. L., & Lubert Stryer. (2020). Protein Structure and Function. Nih.Gov; W H Freeman. https://www.ncbi.nlm.nih.gov/books/NBK21177/
- Types of Proteins. (2016). Retrieved from Utah.edu website: https://learn.genetics.utah.edu/content/basics/proteintypes/
- 14: Translation (Protein synthesis). (2013, November 21). Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Genetics/Book%3A_Working_with_Molecular_Genetics_(Hardison)/Unit_III%3A_The_Pathway_of_Gene_Expression/14%3A_Translation_(Protein_synthesis)
- Translation. (2020, May 3). Genomics. https://serc.carleton.edu/microbelife/research_methods/genomics/translat.html
- Milo, R. (December 2013). “What is the total number of protein molecules per cell volume? A call to rethink some published values”. BioEssays. 35 (12): 1050–55. doi:10.1002/bies.201300066. PMC 3910158. PMID 24114984.
- TANAKA, K. (2009). The proteasome: Overview of structure and functions. Proceedings of the Japan Academy, Series B, 85(1), 12–36. https://doi.org/10.2183/pjab.85.12
© Biology Online. Content provided and moderated by Biology Online Editors.