Redox reaction
n., plural: redox reactions
[ˈɹiːdɒks ɹiˈækʃən]
Definition: In biology, a biochemical reaction wherein the oxidation number of atom(s) has changed
Table of Contents
Redox Reaction Definition
What are redox reactions? This is a common term in chemistry and biology. In chemistry, a redox reaction is one of the types of chemical reaction that involves the alteration in the oxidation states of atoms. In this reaction, there is the actual transfer or shifting of electrons that takes place among different chemical species. In this reaction, one species loses electrons while the other gains the electron. (Olson, 2021) The species which gain the electron is said to be reduced while the species that lost the electron is said to be oxidized. In biology, a redox reaction has defined all aspects of life as it is the form of reaction that occurs in multifarious biological processes.
What is involved in redox reactions? According to the redox reaction rule, the reaction consists of two parts and they always occur together. They are the reduced half and the oxidized half. Reduction-half and oxidation-half reactions are the types of two forms of half-reactions of redox reactions. (MindTouch, 2021)
Redox reaction is a chemical reaction involving both reduction and oxidation, which results in changes in the oxidation numbers of atoms included in the reaction. Oxidation is when there is an increase in oxidation number; reduction is when there is a decrease in oxidation number. It is involved in many important biological processes, such as cellular respiration and photosynthesis. In cellular respiration, for instance, redox reaction occurs when glucose is oxidized to carbon dioxide whereas oxygen is reduced to water. Variant: oxidation-reduction reaction
How to Determine a Redox Reaction?
Redox reaction is always determined by a variation in the oxidation state of two atoms. If there is no alteration in oxidation number then there is no redox reaction. Another feature o a redox reaction is it is comprised of two simultaneous processes, thus, the name. To further understand this, let us understand the definitions of oxidation and reduction. What is oxidation? Oxidation is the process of increasing the oxidation state of an atom, ion, or molecule. Simply put, it means losing the electron. What is reduction? Reduction is the process of decreasing the oxidation state of an atom, ion, or molecule. Or, it simply refers to the gaining of the electron. (Yes, reduction in this regard means “gaining”). So in a redox reaction, while one loses an electron, another gains it.
Types of Redox Reactions
These are the different types of redox reactions and each one is explained below.
- Decomposition Reaction
- Combination Reaction
- Displacement Reaction
- Disproportionation Reaction
1. Decomposition Reaction
As the name indicates, in decomposition reaction, the reactant is split or broken into different components: AB → A + B
Examples:
- 2NaH → 2Na + H2
- 2H2O → 2H2 + O2
The above products are formed by the decomposition of the reactant. As a result, smaller chemical compounds are formed.
However, in some cases, not every decomposition reactions need to be also a redox reaction. For example, CaCO3 → CaO + CO2 is a decomposition reaction but not a redox reaction. Why is this not a redox reaction? The decomposition reaction of CaCO3 → CaO + CO2 involves the dissociation of the components but there is no change in the oxidation states. The oxidation number did not change, which is supposed to change when it is a redox reaction.
2. Combination Reaction
The reverse of the decomposition reaction is a combination reaction. In this reaction, there is a combination of two reactants, and a product is formed: A + B → AB
Examples:
- H2 + Cl2 → 2HClC+O2→CO2
- 4Fe+ 3O2→2Fe2O2
3. Displacement Reaction
As the name indicates, this reaction involves the replacement of an ion or an atom from an element to the ion or atom of another element: X + YZ → XZ + Y . Displacement reactions are of two forms. They are the metal displacement reactions and the non-metal displacement reactions.
- Metal Displacement: In metal displacement reactions, usually, a metal is replaced by another metal. An example is CuSO4+Zn→Cu+ZnSO4. Metal displacement reactions are used in the metallurgical process for obtaining the pure metals from their ores.
- Non-Metal Displacement: In a non-metal displacement reaction, hydrogen H2 or sometimes oxygen O2 is used for displacement.
4. Disproportionation Reaction
In a disproportionation reaction, a single reactant is reducing as well as oxidizing.
Example:
P4 + 3NaOH + 3H2O → 3NaH2PO2 + PH3
Oxidizing and Reducing Agents
In redox reactions, there are two types of chemical agents. They are the oxidizing agents (oxidizers) and the reducing agents (reducers). Let us differentiate them by understanding their role in oxidation and reduction processes.
Table 1: Oxidation vs. Reduction | |
|---|---|
| Oxidation | Reduction |
| In oxidation, the electron is “lost” | In reduction, the electron is “gained” |
| Increased oxidation state of reactants | Decreased oxidation state of reactants |
| A species that donates the electron and undergoes oxidation is known as the reducing agent. Thus, it is also referred to as the “electron donor”. When it loses an electron, it is, thereby, “oxidized”. | A species that accepts the electron or reduces an atom is called an oxidizing agent. Thus, it is also known as the “electron acceptor”. When it accepts an electron, it is, thereby, “reduced”. |
| Examples of reducing agents are electropositive elements, such as sodium, magnesium, and iron | Examples of oxidizing agents are electronegative elements, such as O2 and F2 |
Standard Electrode Potential
Standard Electrode Potential is considered as the value of standard electromotive force (emf) of such a cell in which the hydrogen molecules (H2) are oxidized in the form of solvated protons, under standard pressure.
What is the purpose of redox reactions in the electrochemical cell?
Redox reaction is the basis for an electrochemical cell. It can be divided into two half-reactions, oxidation at the anode and reduction at the cathode. Due to the difference between the electric potential of two electrodes, electricity is generated. And because of the difference among the potentials of two metal electrodes, a difference in the potential is created for the electrolyte. It is used to measure the reducing power of any element or compound.
There is no simple and easy way used to accurately measure the electrode potential or electrolyte potential alone. Changes in pressure, temperature, or concentration not only affect electric potential but also the electrochemistry equations. In a redox reaction, the oxidation potential is the negative of the reduction potential, so it is enough to calculate one of the potentials. That is the reason standard electrode potential is also written as standard reduction potential.
If the value of standard reduction potential is larger, the reduction (gaining of the electron) becomes easier. So for example, the standard reduction potential of F2 is +2.87V and then for Li+, it is -3.05V.
But what about biology? What is the purpose of redox reactions in the biological cell?
In a biological cell, there are different purposes of redox reactions such as during metabolism the sugar molecule is broken down into water, carbon dioxide gas, and energy. There is a transfer of 48 electrons from the carbon atom in sugar to oxygen along with the release of energy.
C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) + energy
Another purpose of redox reactions in the living body is cellular communication. The reactive molecules containing oxygen in the cell play a role as signaling molecules. For example, reactive molecules containing oxygen (O2, H2O2, NO) are produced in the cell in a controlled manner during redox reactions. These chemicals have different roles such as wound healing, inflammation, aging, and programmed cell death.
According to newer studies, the redox reaction can also be exploited in the cells for cancer treatments. A class of cancer treatment drugs enhances the production of reactive molecules containing oxygen in the tumor body which eventually kills the cancer cells.
Examples of Redox Reactions
Some examples of redox reactions are as follows.
Example 1: Reaction between Hydrogen and Fluorine
In the reaction of fluorine and hydrogen, oxidation takes place at hydrogen while reduction occurs at fluorine. Hydrogen and fluorine combine and form Hydrogen Fluoride.
The following equation shows the reaction: H2 + F2 → 2HF
The oxidation equation is H2 → 2H+ + 2e–
The reduction equation is F2 + 2e– → 2F–
Example 2: Reaction between Iron and Hydrogen Peroxide
Hydrogen peroxide oxidizes ferrous ion Fe2+ into ferric ion Fe3+ in the presence of an acid. As a result, a hydroxide ion is formed. Hydrogen peroxide reacts with a proton, which is donated by the acid for the formation of water.
2Fe22+ + H2O2 + 2H+ → 2Fe3+ + 2H2O
Oxidation half-reaction is Fe2+ → Fe3+ + e–
Reduction half-reaction is H2O2 + 2e– → 2 OH–
Example 3: Reaction between Zinc and Copper
When Zn displaces the ion of copper in the solution of copper sulfate, copper metal is gained.
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
The oxidation half-reaction is Zn → Zn2+ + 2e–
The reduction half-reaction is Cu2+ + 2e– → Cu
Significance of Redox Reactions
Oxidation-reduction reactions are significant as they are the foremost and chief source of energy on Earth either in natural (biological) or non-natural (artificial) ways. A huge amount of energy can be gained in an oxidation reaction either by the removal of hydrogen or by the combination of oxygen. (Chemistry, 2021)
Redox Reactions in Industry
Many chemicals that are commonly used in industries like chlorine, caustic soda, etc are formed by redox reactions. Redox reactions are used in bleaching material and water sanitizing. In the manufacturing of industrial cleaning products, the oxidation process is used. Many metals that are at risk of corrosion are protected by joining them with sacrificial anodes. Galvanization of steel is an example of it. The oxidation of ammonia produced nitric acid, which is an essential fertilizer. Redox reaction is also used for the separation of metals from their ores. Smelting of metal sulfide in the existence of a reducing agent is also an example of this. In the manufacturing of gold-plated ornaments, a redox reaction is used to apply a thin coat of material on the surface of the object. This process is also known as electroplating. (W3spoint.com, 2021)
Redox Reactions in Biology
What is the purpose of redox reactions in the cell? Many biological processes involve redox reactions, such as in cellular respiration and photosynthesis.
Cellular respiration
Cellular respiration (C6H12O6 + 6 O2 → 6 CO2 + 6 H2O) is the oxidation of glucose into carbon dioxide (CO2) and reduction of oxygen (O2) to water (H2O). The method of cellular respiration redox is related to the reduction and oxidation of NAD+ into NADH and vice versa. Below is a schematic diagram of cellular respiration.


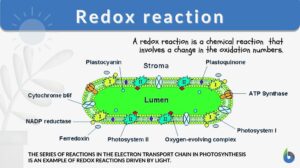
Photosynthesis
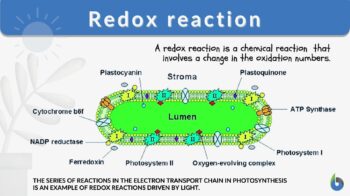
In redox reactions in photosynthesis (6 CO2 + 6 H2O + light energy → C6H12O6 + 6 O2), Carbon dioxide is reduced into sugar and water oxidation gives molecular oxygen. The number of electrons in oxygen is 8. Although cellular respiration and photosynthesis appear like opposite reactions, these two processes are not reverse of each other.
READ: Photosynthesis – Photolysis and Carbon Fixation
Redox Cycling
An extensive variety of aromatic compounds are reduced with the help of enzymes for the formation of free radicals. Free radicals have one or more electrons than their parent. The electron donor can be any flavoenzyme or its coenzyme. Once after formation, the free radicals in the form of anions reduced the oxygen into superoxide, and also unaffected parent (original) compound get renewed. Overall, in this reaction, there is oxidation at flavoenzyme coenzyme and reduction at molecular oxygen for the formation of superoxide. This catalytic behavior is termed Redox Cycling.
Redox Reactions in Geology
In geology, redox reaction has many uses like:
- Mobilization of minerals
- Formation of minerals
- Depositional environments
In the color of rocks, the redox state is visible. The oxidizing rock had a red color. When a reducing liquid or fluid is passed to the rock it gives green or white color. The reducing liquid or fluid had uranium-bearing minerals. Moqui marbles and uranium deposits are some examples of deposits formed from geologic redox reactions.
Redox Reactions in Soils
In a redox reaction, there is simultaneous oxidation and reduction reaction. An example of a redox reaction in the soil is the oxidation of ferrous to ferric iron by the reduction of oxygen in the presence of water. (Nature, 2021)
Balancing Redox Reactions
The following explains how to balance a redox equation or how to do redox reactions or redox reactions in basic solutions.
- Step I: Write the unbalanced equation.
- Step II: Isolate the redox reaction into two half-reactions
Give oxidation number to each atom
Find and write the redox couples
Combine the redox couples into two half-reactions - Step III: Balancing half-reactions
Balance all atoms except H2 and O2
Balance all O2 atoms with water H2O
Balance the atoms of hydrogen with H+
Add 1 OH– on each side of H+ for a basic medium - Step IV: Balance the number of electrons
- Step V: In half-reaction, make electron loss equal to electron gain
- Step VI: Together add half-reactions
- Step VII: Simplify the equation
At the end check all the charges and elements are balanced. For easiness, an online redox reaction calculator or oxidation state calculator is also used for equation balancing.
How to find oxidation numbers? Following are some oxidation number rules:
1. Zero is the oxidation number of free elements.
2. The charge on an ion is equivalent to the oxidation number of mono-atomic ions.
3. The charge on ions is also equivalent to the oxidation number of polyatomic ions.
4. The oxidation number of Hydrogen is +1, however, when it is in a compound with some electronegative element it is, oxidation number changes to -1.
5. The oxygen oxidation number is -2 but in peroxides, it is -1.
6. The carbon oxidation number varies widely. In CH4 it is -4 while in CO2 it is +4
7. Group 1 element had oxidation number +1.
8. Group 2 element had oxidation number +2.
9. Group 17 element had oxidation number -1.
10. In a neutral compound, the total oxidation number of all atoms is zero.
Try to answer the quiz below to check what you have learned so far about redox reactions.
References
- VRChemistry. (2021). Redox Reactions and Electrochemical Potential. Retrieved 04 Nov, 2021, from https://vrchemistry.chem.ox.ac.uk/potential/text/redox1.htm
- Generalic, E. (2021). BALANCING REDOX REACTIONS. Retrieved 04 Nov, 2021, from https://www.periodni.com/half-reaction_method.php
- MindTouch, P. b. (2021). Oxidation-Reduction Reactions Retrieved 04 Nov, 2021, from https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions
- Nature, S. (2021). Redox Reactions and Diagrams in Soil. Retrieved 04 Nov, 2021, from https://link.springer.com/referenceworkentry/10.1007%2F978-1-4020-3995-9_477
- Olson, M. V. (2021). oxidation-reduction reaction. Retrieved 04 Nov, 2021, from https://www.britannica.com/science/oxidation-reduction-reaction/General-theory
- W3spoint.com. (2021). Redox Reaction in Electrochemistry. Retrieved 04 Nov, 2021, from https://www.w3spoint.com/
- Socratic.org. (2021). Calculate the oxidation number of an element in a compound. Retrieved 04 Nov, 2021, from https://socratic.org/questions/how-do-you-calculate-the-oxidation-number-of-an-element-in-a-compound
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.