Reducing Sugar
n., plural: reducing sugars
Definition: a sugar that serves as a reducing agent
Table of Contents
Reducing Sugar Definition
What is reducing sugar? The type of sugar that acts as the reducing agent and can effectively donate electrons to some other molecule by oxidizing it is called reducing sugar. In another definition, any sugar that tends to act as the reducing agent since it has either an aldehyde group (-CHO) or the ketone group (-CO-) is called reducing sugar.
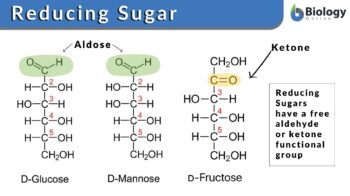
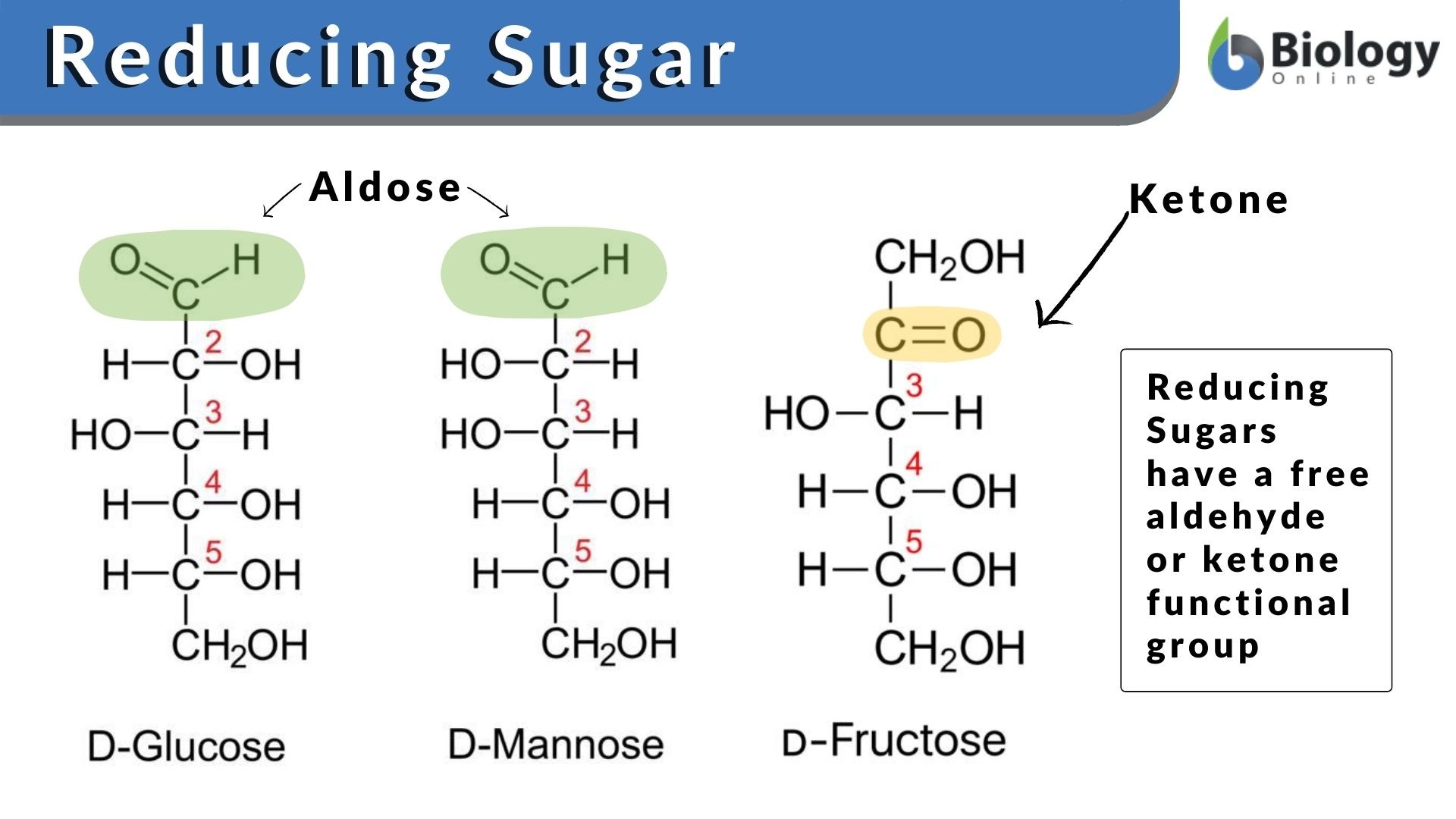
Reducing sugar comes under the category of carbohydrate or natural sugar but it consists of either a free aldehyde group or a ketone group. The sugar structure with a free aldehyde or the ketone group is called the reducing end of sugar. The end of the molecule with the free anomeric carbon is referred to as the reducing end.
Some of the disaccharides, oligosaccharides, polysaccharides, and all monosaccharides are reducing sugars. The monosaccharides are categorized into two groups: (1) aldoses which contain the free aldehyde group and (2) ketoses where there is a ketone group. It should be remembered here that before acting as the reducing agents, ketoses must tautomerize aldoses. The structural isomers of the chemical compounds that can instantly interconvert are tautomers and the process in chemistry is referred to as tautomerization. (Ref. 1)
Is glucose an aldose?
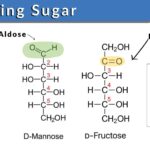
The most common example of ketose is fructose whereas glucose and galactose are aldoses. The chemical formulation of sugar is Cn (H2O) n (e.g., C6H12O6 for glucose), which is naturally found in all fruits, dairy products, vegetables, and whole grains. The chemical configuration and structure of sugar particularly, glucose, fructose, and sucrose have been elaborated in Figure 1. These sugars are the carbohydrates that we often consume in our diet. Single sugar molecules (monomers) are the monosaccharides and the two monomers linked together are the disaccharides. Relatively larger chains of sugar molecules that are interconnected with each other via chains are oligosaccharides and polysaccharides.
The three most common disaccharide examples are lactose, sucrose, and maltose. Cellulose, starch, glycogen, and chitin are all polysaccharides examples.
Why is sucrose not a reducing sugar?
Sucrose is a nonreducing sugar. In sucrose, there are glycosidic bonds between their anomeric carbons to retain the cyclic form of sucrose, avoiding its conversion into the form of an open chain with an aldehyde group. Contrarily, maltose and lactose, which are the reducing sugar, have a free anomeric carbon that can get converted into an open-chain form by forming a bond with the aldehyde group.
Is starch a reducing sugar? It should be remembered here that starch is a non-reducing sugar as it does not have any reducing group present.
Reducing vs. Non-reducing Sugars
What are reducing sugar and nonreducing sugar?
Any carbohydrate that is capable of causing the reduction of some other substances without being hydrolyzed first is the reducing sugar whereas sugars that do not possess a free ketone or an aldehyde group are called the non-reducing sugar. It is worth mentioning here that the non-reducing sugars never get oxidized. The most common example of non-reducing sugar is sucrose. The very important question that needs to be addressed here is this: why sucrose is a non-reducing sugar? The reason is that in sucrose the two units of monosaccharides units are held together very tightly by the glycosidic linkages between the C-2 carbon of the fructose and the C-1 of glucose. Since the reducing groups of fructose and glucose are involved in the glycosidic bond formation, sucrose, therefore, is a non-reducing sugar.
Key differences between reducing and non-reducing sugars:
- Reducing sugars are the carbohydrates with free aldehyde and the ketone group while in the non-reducing sugar, no such free groups are found; rather, they are available in the formation of bonds.
- The non-reducing sugar form is in the acetal or the ketal form whereas the reducing forms are in the hemiketal or the hemiacetal.
- The reducing sugars possess mutarotation while on the other hand, the non-reducing never exhibit such rotational behaviors.
- The reducing sugar forms osazones while the other form of sugar doesn’t form osazones.
- The reducing sugars are mainly monosaccharides whereas all polysaccharides are non-reducing sugars.
- The reducing sugar can reduce the capric ions of the Fehling or the Benedict solution into the cuprous ions whereas, the reduction of cupric ions into the cuprous ions is not achieved in the non-reducing sugars.
- The most common examples of reducing sugar are maltose, lactose, gentiobiose, cellobiose, and melibiose while sucrose and trehalose are placed in the examples of non-reducing sugars.
Characteristics of Reducing Sugars
The reducing sugar is also mentioned as a compound, such as sugar or an element, for instance, calcium that loses an electron to another chemical or biological species in the reactions stated as the oxidation-reduction (often abbreviated as the redox reactions). Some of the most significant characteristics of reducing sugar have been summarized in the points below. (Ref. 2)
- The reducing sugars such as glucose and fructose have a free aldehyde group and ketone in their structures, respectively.
- The reducing sugar mostly forms a hemiacetal structure where a carbon gets attached to a couple of oxygen atoms thus resulting in the formation of either ether or alcohol.
- The reducing sugars can be oxidized with some relatively mild oxidizing agents such as salts of metals.
- In an aqueous solution, the reducing agents generally generate one or more compounds comprising an aldehyde group.
- In the Maillard reactions, the reducing sugars react with the amino acids, and a series of chemical and biological reactions occur. The Maillard reaction is the process in which amines react with the reducing sugars resulting in the browning of the food. The reaction normally occurs when either the food is left at room temperature for a long period or is heated. The browning of food can be often seen on the crust of the bread or the skin of roasted dietary items. Similarly, the aroma smelled in coffee, bread, chocolate, and baked item is due to the contribution of the Maillard reaction.
- The reducing sugars produce mutarotation and form osazones.
Oxidation-Reduction Process
What is reduction? The loss of electrons during a reaction of a molecule is called oxidation while the gain of single or multiple electrons is called reduction. The oxidation and reduction reactions (also called redox reactions) are chemical reactions in which the oxidation number of the chemical species that are taking part in the reaction changes. The redox processes are the wide range of reactions that include the majority of the chemical and biological processes taking part around us. Rusting and dissolution of the metals, browning of the fruits, fire reactions, respiration, and the process of photosynthesis are all oxidation-reduction processes.
The redox reactions involve the transfer of hydrogen, oxygen, or electrons where two very important characteristics are common in all three reactions. Firstly, they are “coupled”, which means that in any oxidation reaction, there is a sideway reduction reaction. Secondly, they always involve a net chemical change where new substituents are formed by the reaction of reactants. The examples of all three forms of chemical reaction have been elaborated on below. (Ref. 3)
Oxygen atom Transfer:
C+ 2H2O → CO2 + 2H2
Hydrogen atom Transfer:
N2H4+ O2 → N2+ 2H2O
Electron Transfer:
Zn + Cu+2 → Zn+2 + Cu
Tests for Analyzing the Presence of Reducing Sugar
Two very important tests are often performed to identify the presence of reducing sugar. These tests are the Benedict test and the Fehling test. (Ref. 4)
Benedict Test
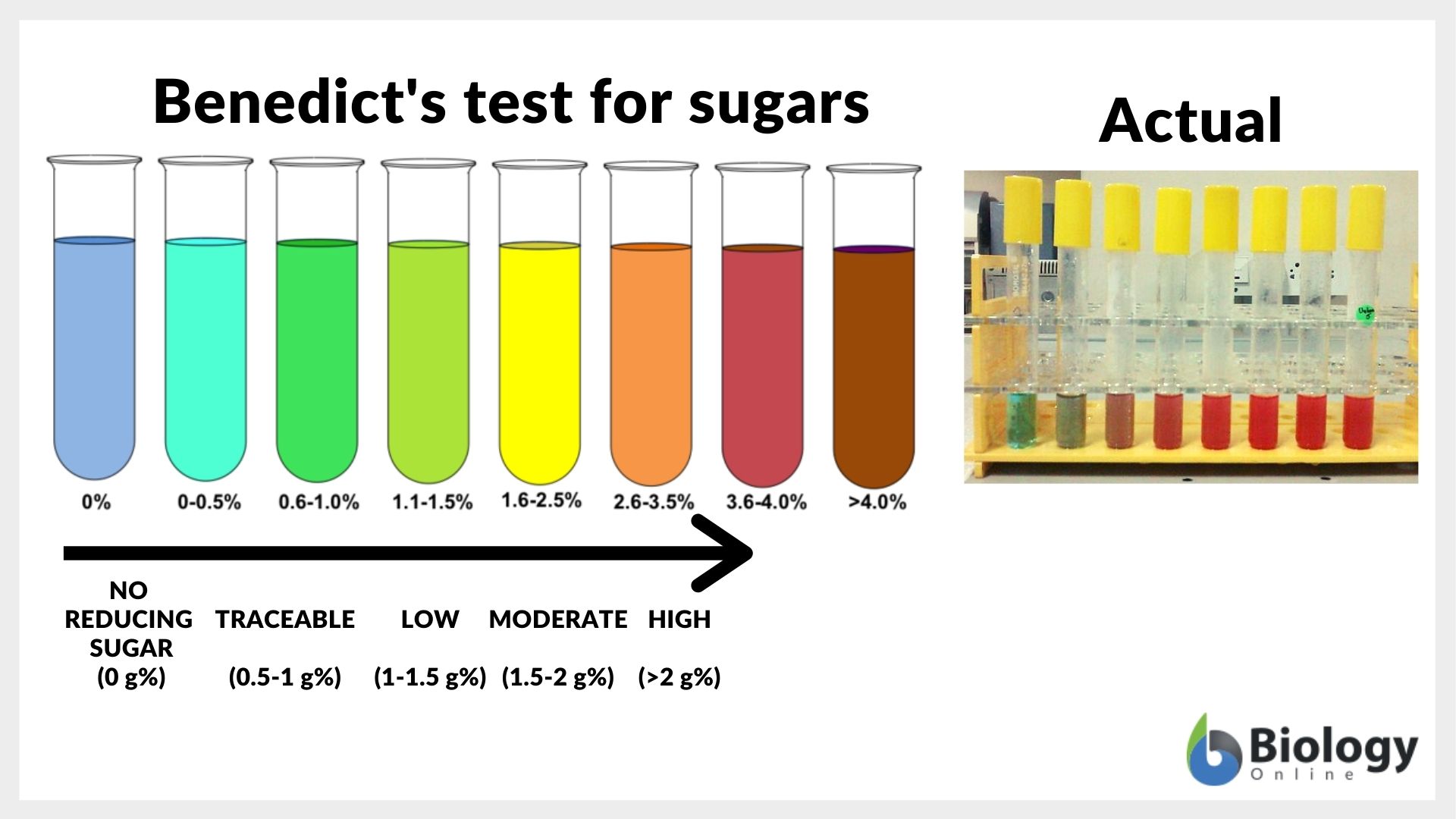
In the Benedict test, the food samples from which the presence of reducing sugar has to be detected are dissolved in water, and after this, a very small amount of Benedict’s reagent is added after which the solution begins to cool down. After around ten minutes the solution starts to change its color. If the color changes to blue it means that there is no reducing sugar present. But if the color changes to green, yellow, orange, red, and then finally to dark red or brown color confirms the presence of reducing sugar in the food. The chemical composition of the Benedict solution states that it is made of an anhydrous solution of sodium citrate, sodium carbonate, and copper II sulfate pentahydrate. During its reaction with the reducing sugar, the blue copper sulfate in the solution is converted into red-brown copper sulfide. It is worth mentioning here that these tests only show the qualitative analysis of reducing sugar.
Modified by Maria Victoria Gonzaga of Biology Online, from the works of Thebiologyprimer (A)- (diagram) and Psgs123xyz (B) – (diagram), both in CC0 license.
Fehling Test
In the Fehling test, the solution is warmed until the sample where the availability of reducing sugar has to be tested is homogeneously mixed in water after which the Fehling solution is added. If the reducing sugar is present the color of the solution will be changed to a red precipitate color resembling rust.
Fehling’s solution is made by mixing equal amounts of aqueous solutions of copper II sulfate pentahydrate and potassium sodium tartrate tetrahydrate. This test is specifically used for the identification of monosaccharides, especially ketoses and aldoses. These tests can be used in the laboratory for the determination of reducing sugar present in the urine which can be used to diagnose diabetes mellitus.
Similarly, another group of reagents often used to determine the presence of functional groups of aldehydes and aromatic aldehydes with some of the alpha-hydroxy ketones that can be tautomerized into aldehydes is Tollen’s reagents, and the test that is performed is called Tollen’s test. The tollen’s reagent is an alkaline solution of ammoniacal silver nitrate. It must be noted here that the reduction of aldehydes results in the formation of primary alcohols while the reduction of ketones gives secondary alcohols.
Reducing Sugar Examples
The most common example of reducing sugar and monosaccharides is glucose. In the human body, glucose is also referred to as blood sugar. It is essential for the proper functioning of the brain and as a source of energy in various physical activities. Another reducing sugar is fructose, which is the sweetest of all monosaccharides. Galactose is another example of reducing sugar. It is a component of lactose available in many dairy products. Moreover, the list of reducing sugars also includes arabinose and glyceraldehyde.
Biological Importance of Reducing Sugars
Carbohydrates, especially reducing sugar are the most abundant organic molecules that can be found in nature. They have a wide range of functions in biology. They provide a significant fraction of daily used dietary calories in most of the living organisms living on the earth. Also, their major role is to act as the storage of energy in living bodies.
Read: Glycolysis, Fermentation, and Aerobic respiration
Carbohydrates also serve as one of the cell membrane components and function primarily in mediating various intermolecular communications in the bodies of living organisms. Lastly, via Maillard reactions, carbohydrates are responsible for determining the crust color and the taste of the food such as coffee, bread, and roasted food items.
Uses of Reducing Sugars
Reducing sugar has many uses in our daily life activities. In medicines, the Fehling solution has been used as a test to detect diabetes in human blood. The relative measurement of the number of oxidizing agents reduced by the available glucose makes it easy to calculate the concentration of glucose present in human blood or urine. Moreover, after the calculation of the exact amount of glucose present, it becomes easier to prescribe the amount of insulin that must be taken by the patients from the doctors. In food chemistry, the levels of reducing sugar in the products such as wine, juices, and sugar cane decide their quality. (Ref. 5)
NOTE IT!
“The Role of Reducing Sugars in Enhancing Flavor and Color in Baked Goods: A Dive into Food Science“
Simple sugars and complex carbohydrates have been vital ingredients in many dishes as they serve as an important energy source. Sucrose, which emerged as a prominent reducing sugar, has long been a culinary staple and a common sweetening agent that we simply refer to it as the “common table sugar”. Let’s dive a little further to explore the role of reducing sugars in enhancing flavor, color, and the overall appeal of baked goods.
The Maillard browning effect:
Reducing sugars can cast that delightful browning and aroma in baked goods. Reducing sugars possess free amino groups that are essential in enabling the browning reaction (also called the Maillard reaction). The open-chain form of reducing sugars, in particular, enables them to engage in this complex chemical reaction, which occurs between the amino group (-NH2) and the carbonyl group (C=O) of a reducing sugar when exposed to heat. The reaction leads to the formation of melanoidins, which are responsible for the browning characteristic as well as the aroma and flavor of foods that develop by cooking techniques (such as baking, frying, toasting, searing, etc.).
Delicious yet healthy:
While melanoidins and other flavor-enhancing chemicals are produced during the Maillard reaction, other chemical compounds deemed as “unhealthy” are also produced. For instance, acrylamide, a potential carcinogen, is a byproduct of the reaction between asparagine (an amino acid) and reducing sugars in food that is cooked at high temperatures.
Consequently, food scientists are on the constant lookout for cooking techniques and processing methods that will help minimize acrylamide levels in foods while trying to achieve Maillard browning effects and flavor development so as not to compromise the flavor and taste of foods as well as their nutritional value.
One such method involves the use of aqueous ammonia. This process targets the free amino groups in sugars, leading to their transformation into unreactive forms. As a result, the sugars become less available for browning reactions, consequently reducing the overall sugar impact on the final product’s color and flavor.

Try to answer the quiz below to check what you have learned so far about reducing sugar.
References
[1] Rizzo, N. (2011, February 21). “The Definition of Reducing Sugars,” livestrong.com.https://www.livestrong.com/article/386795-the-definition-of-reducing-sugars/
[2] Gunawardena, G. (2016, January 4). Reducing Sugar. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Ancillary_Materials/Reference/Organic_Chemistry_Glossary/Reducing_Sugar
[3] Moghaddam, S. V., Rezaei, M., & Meshkani, F. (2019). Surfactant‐Free Sol–Gel Synthesis Method for the Preparation of Mesoporous High Surface Area NiO–Al 2 O 3 Nanopowder and Its Application in Catalytic CO 2 Methanation. Energy Technology, 8(1), 1900778. https://doi.org/10.1002/ente.201900778
[4] Kelly, M. Test for Reducing Sugars. (2018). Sciencing. https://sciencing.com/test-reducing-sugars-5529759.html
[5] Reducing Sugar | Baking Ingredients | BAKERpedia. (2020, July 30). BAKERpedia. https://bakerpedia.com/ingredients/reducing-sugar/
©BiologyOnline.com. Content provided and moderated by BiologyOnline Editors.