Saccharide
n., plural: saccharides
[ˈsæk.ɚ.ɪd]
Definition: The unit of carbohydrates
Table of Contents
Saccharide Definition
What is a saccharide molecule? A saccharide is the unit structure of carbohydrates. In biochemistry, saccharides are the carbohydrates or sugars that serve as the main source of energy that fuels diverse biochemical processes.
The chemical formula for carbohydrates is Cn(H2O)n, this formula indicates whether the saccharide is simple or complex. Saccharide molecules generally consist of either five or six carbon atoms. Moreover, saccharides may be found either in a ring shape or a short-chain.
What are carbohydrates? Carbohydrates are organic compounds that may be either ketones or aldehydes with extra hydroxyl groups, which are added to every carbon of the skeleton of aldehydes or ketones structures, except one carbon, which is called the carbonyl group, to form carbohydrates.
Simple saccharides have two essential groups; the first reactive group is carbonyl group, which consists of a carbon group with a double bond and an oxygen group from one side. The same carbon interacts with two different carbon or hydrogen groups from the other two sides. The second reactive group is the hydroxyl group that interacts with carbons on the chain except with that of the carbonyl group.
Plant and animal cells consist mainly of carbohydrates. It is the most important organic compound found in the cells of living organisms since carbohydrates form about 40% of the dry weight of plants. On the other hand, the cell wall structure in plant cells is composed mainly of carbohydrates.
Carbohydrates are not only included in the structure of plant and animal cells, they are also consumed by plants and animals to produce energy that enables plants and animals to do their activities. As carbohydrate molecules form about 43% of calories consumed by humans. On other hand, saccharides or carbohydrates are used in pharmaceutical applications to produce antibiotics like tetracyclines and D-glucose solution, which is known as dextrose. In addition, sucrose is also used to produce sweeteners. Therefore, sugars and starches are examples of saccharides and biologists define saccharide as the main source of energy.
A saccharide is the unit structure of carbohydrates. Examples are monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Carbohydrates are simple organic compounds that are aldehydes or ketones with many hydroxyl groups added usually on each carbon atom not part of the aldehyde or ketone functional group. The general chemical formula of carbohydrates is Cn (H2O) n. Not all carbohydrates follow this formula and are slightly different in structure from this rule. There are also compounds that seem to follow this rule but are not carbohydrates (e.g. formaldehyde).
Main functions:
Saccharides are an essential structural component of living cells. They are an important source of energy for animals.
Types:
They may be classified according to the number of monomeric units that comprise them: monosaccharides, disaccharides, oligosaccharides, polysaccharides, and heterosaccharides. Monosaccharides are the most fundamental type is the sugars. They are glucose, galactose, and fructose. These simple sugars can combine with each other to form more complex types. The combination of two simple sugars is called a disaccharide whereas those consisting of two to ten simple sugars are called oligosaccharides, and those with a larger number are called polysaccharides.
Saccharide Structure
The general formula of saccharide molecules is Cn (H2O) n. However, not all saccharides follow this formula, but simple structures do, such as glucose and fructose.
The number of carbon units indicates the type of saccharides. Monosaccharides have one carbon atom. If two monosaccharide units join, the saccharide formed is called a disaccharide. Trisaccharides are formed of three monosaccharide units. Disaccharides or trisaccharides are formed by linking two or three monosaccharides by acetal or ketal linkage. In addition, if the number of monosaccharides units is between 4 to 10 atoms, the saccharide is called an oligosaccharide. If the number of carbons exceeds 10 atoms, this type is referred to as polysaccharides.
The structure of each type is formed of:
A. Monosaccharides are referred to as simple sugars. Where no hydrolysis can happen to this type of saccharides. Structurally, Monosaccharides may be classified in two ways. The first way is based on the number of carbon. For example, a triose consists of three carbon atoms, tetrose has four carbon atoms, pentose has 5 carbon atoms, and hexose is composed of 6 carbon atoms. The second way is according to the fundamental or reactive group into aldose that has an aldehyde as the active group and ketose (meaning a reactive group is a ketone group).
Monosaccharides may be found either in straight chains or ring form. The ring form is found in biological systems such as in plants, animals, and humans. Whereas the straight chain is found in aqueous systems. Glucose which is the main source of energy for the human body may be found in two isomers, alpha, and beta. The two isomers differ in the direction of the hydroxyl group.
B. Disaccharides are two identical or non-identical monosaccharides that are linked together by a glycosidic covalent bond. The main structural difference between disaccharides and monosaccharides is in the presence of the glycosidic bond.
C. Oligosaccharides consist of 3 to 10 linked identical or non-identical units of monosaccharides. This type of saccharides is found mainly in beans and used in growing some microbes.
D. Polysaccharides are formed of 10 monosaccharides or more. Polysaccharides can be formed by linking polymers of simple monosaccharides such as starch. Polysaccharides may be homopolysaccharides or hetero-polysaccharides according to the similarity of units in polymers forming the polysaccharides. As homopolysaccharides consist of one type of saccharide polymer such as starch which consists of polymers of D-glucose. Unlike homopolysaccharides, Hetropolysaccharides consist of different units of polymers of monosaccharides such as pectin and hemicellulose in plant cell walls.
Let us find out more about the types and subtypes of these saccharides below.
Types of Saccharides
There are four fundamental types of saccharides varying from the simplest ones such as monosaccharides to the complex ones such as polysaccharides.
1. Monosaccharides
Mono refers to one and saccharides mean sugar. The most common monosaccharides are glucose, galactose and fructose.
- Glucose is the most common monosaccharide which consists of 6 carbon atoms. It is known as an aldohexose compound; its molecular structure is C6H12O6. Glucose may be found in straight chains in aqueous media or as rings in biological systems. Glucose is the main source of energy for humans. The glucose is metabolized by the human body into ATP and CO2 in case of oxygen sufficiency. The glucose normal range in the blood is between 70 to 120 mg per 100ml. The liver controls the level of glucose in the blood by releasing two hormones from two types of cells; alpha and beta. Insulin, which is released by beta cells, controls excess glucose in the blood and stores it as glycogen in the liver and muscles. The other hormone is glucagon, which is released by alpha cells, it controls the shortage or decrease of glucose in the blood by increasing its level. The normal range of glucose in the blood is controlled by insulin and glucagon hormones. When the liver is unable to control the glucose level, a common medical condition appears which is diabetes. Diabetes has two types: Type 1 diabetes and type 2 diabetes. If this condition is not controlled, it may progress to acute circulatory failure and death. In plants, Excess Glucose is stored as starch to be used when energy sources are insufficient. Monosaccharides interact with the hydroxyl group of water, so they are generally water-soluble molecules, especially glucose. They are found as crystalline particles.

Figure 1: glucose structure - Galactose is an epimer of glucose. It has a similar molecular formula to glucose. Galactose is identical to glucose in structure. But there is a difference in the direction of the hydroxyl group in the carbon number 4 of the carbon skeleton. This difference creates different physical and chemical properties between them. Galactose in humans can be converted by the liver into glucose to be used as a source of energy if there is an insufficient amount of glucose. Galactose can be found in two conformational states –‘boat’ and ‘chair’. Those two conformations control the stability of galactose in aqueous solutions. The most common dietary source of galactose is lactose which consists of glucose and galactose. Lactose is the sugar of milk (see Disaccharides below).
- Fructose is an isomer of glucose. They have identical chemical formulas but are different in their three-dimensional structure. Since fructose has a ketone functional group. Conversely, glucose has an aldehyde group. This makes glucose with 6 carbons skeleton whereas fructose has only 5 carbons skeleton. The liver converts fructose into glucose to be used as another source of energy. Fruits are the main source of fructose.
2. Disaccharides
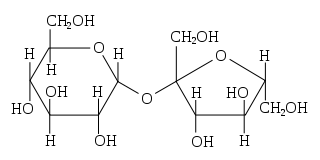
Disaccharides are a type of saccharides that consist of two linked monosaccharides by a bond called a glycosidic bond. When the two monosaccharides are linked, one molecule of water is released. Sucrose and lactose are the most common examples of disaccharides.
- Lactose consists of one glucose and one galactose molecule. The type of glycosidic bond is Beta type. This bond cannot be digested by some humans. This condition is known as lactose intolerance, which leads to bloating.
- Sucrose consists of one glucose and one fructose molecule. The linkage between them is an alpha-type glycosidic bond. Sucrose is the best preservative as it has no reactive ends over other saccharides.

Figure 2: disaccharide structure
3. Oligosaccharides
Oligosaccharides are a type of carbohydrates that are composed of more than two units of monosaccharides. One of the most common examples of this type is raffinose. Raffinose is composed of repeating units of glucose, galactose, and fructose. The bond between them is unbreakable by the small intestine and can only be broken in the large intestine by bacteria.
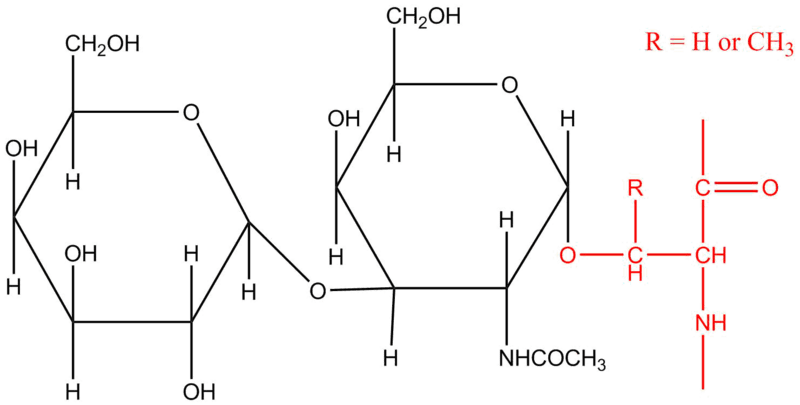
4. Polysaccharides
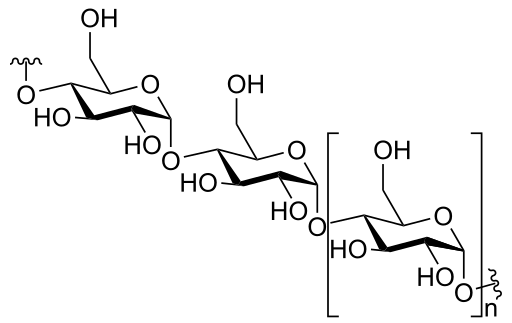
Polysaccharides are complex sugars. As polysaccharides, They consist of repeating units of monosaccharides. The most common example is starch which is considered the main form of stored glucose in plants, it is used to generate energy when there are insufficient energy sources. Starch has two forms — amylose and amylopectin. The main difference between them is that amylose is straight-chain and amylopectin is a highly branched polysaccharide.
Saccharide Examples
The most common examples of saccharides are:
- The most abundant monosaccharides are pentoses (for example, xylose is found mainly in plants as xylan in the wood substance) and hexoses (such as glucose and fructose, which are the most common source of carbohydrates in animal tissues).
- The most common example of disaccharides is sucrose (which consists of two monosaccharides — glucose and fructose) and lactose (milk sugar), and maltose.
- Oligosaccharides such as raffinose consist of three types of sugars — glucose, fructose, and galactose.
- The most common example of polysaccharides is starch (common in plants) and glycogen (common in animals).
Answer the quiz below to check what you have learned so far about saccharides.
References
- Bemiller – Primary Reference Book – wiley online library. (n.d.). Retrieved April 12, 2022, from https://onlinelibrary.wiley.com/doi/full/10.1002/0471238961.0301180202051309.a01.pub2
- Cherian, G. (2019, April 14). III. Carbohydrates, structures and types. A Guide to the Principles of Animal Nutrition. Retrieved April 12, 2022, from https://open.oregonstate.education/animalnutrition/chapter/chapter-3/
- Encyclopædia Britannica, inc. (n.d.). Glucose. Encyclopædia Britannica. Retrieved April 13, 2022, from https://www.britannica.com/science/glucose
- Galactose. Galactose – an overview | ScienceDirect Topics. (n.d.). Retrieved April 13, 2022, from https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/galactose
- Person. (2017, March 24). What is glucose and what does it do? Healthline. Retrieved April 13, 2022, from https://www.healthline.com/health/glucose#how-glucose-works
- Saccharide. Biology Articles, Tutorials & Dictionary Online. (2021, July 21). Retrieved April 12, 2022, from https://www.biologyonline.com/dictionary/saccharide
- SparkNotes. (n.d.). Sparknotes. Retrieved April 13, 2022, from https://www.sparknotes.com/health/carbohydrates/section1/
- SparkNotes. (n.d.). Sparknotes. Retrieved April 13, 2022, from https://www.sparknotes.com/health/carbohydrates/section1/page/2/
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.