second-order kinetics
A term describing the reaction rate of a chemical reaction in which the rate is proportional to the product of the concentrations (in moles) of two of the reactants (also called bimolecular kinetics), or to the square of the molar concentration of the reactant if there is only one. Such a reaction might have an equation like rate = kor rate = kA2, where k is the reaction rate constant, is the concentration of reactant A, and B is the concentration of reactant B.
Dictionary > Second-order kinetics
You will also like...

The Evolutionary Development of Multicellular Organisms
Multicellular organisms evolved. The first ones were likely in the form of sponges. Multicellularity led to the evolutio..
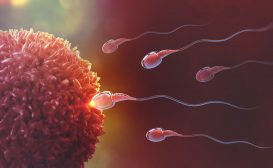
Human Reproduction and Fertilization
For human species to obviate extinction, reproductive mature adults should be producing viable offspring in order to con..

Roots
This study guide tackles plant roots in greater detail. It delves into the development of plant roots, the root structur..
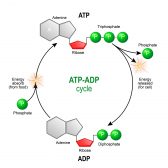
ATP & ADP – Biological Energy
ATP is the energy source that is typically used by an organism in its daily activities. The name is based on its structu..
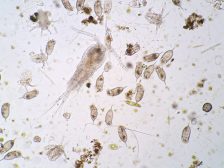
Freshwater Communities & Plankton
Planktons are microscopic organisms that live suspended in aquatic habitats. There are two groups: the phytoplanktons an..

Leaves
Leaves are the major photosynthetic organ of a plant. Apart from that, they are also crucial to water movement. In this ..

