Spermiogenesis
n., plural: spermiogeneses
[ˌspɜːmɪəʊˈdʒɛnɪsɪs]
Definition: the stage of spermatogenesis wherein the spermatids differentiate into mature spermatozoa
Table of Contents
Spermiogenesis Definition
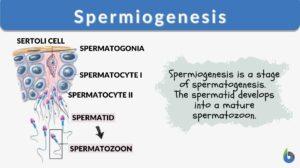
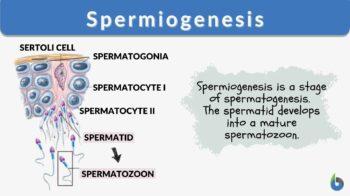
Spermiogenesis is the stage of spermatogenesis wherein the spermatids differentiate into mature spermatozoa. Prior to this stage, the germ cells go through a series of cellular divisions, particularly mitosis (spermatocytogenesis) and meiosis (spermatidogenesis).
In brief, the male primordial germ cells called spermatogonia divide mitotically to produce more spermatogonia. Some of these cells will stop dividing and become spermatocytes that will enter meiosis to produce haploid spermatids. Still bulky and rounded, the spermatids enter spermiogenesis. During this stage, the spermatids undergo morphological changes to become compacted and streamlined spermatozoa (sperm cells). These cells will include an acrosome atop the compacted head, the midpiece containing a ring of mitochondria, and a flagellum for motility.
Spermiogenesis is comprised of four phases. These phases are (1) Golgi phase, (2) Cap phase, (3) Tail phase, and (4) Maturation phase. Following spermiogenesis is spermiation. The spermatozoa are released from the seminiferous tubules to migrate to the epididymis where they become fully- differentiated motile spermatozoa. Disturbances in any of these phases could give rise to sperm cells with abnormal morphology, which in turn could affect male fertility.
Etymology
The term spermiogenesis, similar to the term spermatogenesis, is derived from the Greek words sperma (meaning “seed”) and genesis (meaning “birth”, “origin”, “creation”). Synonyms: spermiohistogenesis; spermateliosis.
Basic concepts
To gain a better grasp of spermiogenesis, a review of the concepts pertinent to the subject could be useful. Germ cells are biological cells that give rise to sex cells or gametes. During embryonic development, the germ cells from the primitive streak migrate to the developing gonads. In humans, the production of male gametes begins at puberty. The process is called spermatogenesis. It occurs in the seminiferous tubules of the male gonad (testis).
In mammals, the male germ cells include the spermatogonium, the spermatocyte, and the spermatid. The spermatogonium (plural: spermatogonia) is an undifferentiated, mitotically active germ cell. It is located next to the basal lamina. The spermatocyte is the male gametocyte that arises from a spermatogonium. This germ cell undergoes meiosis. The spermatocyte that carries out meiosis I is called primary spermatocyte whereas the spermatocyte that goes through meiosis II is called secondary spermatocyte. The primary spermatocyte is the diploid (just like the spermatogonium). The secondary spermatocytes are haploid. The spermatid is the product of a spermatocyte after meiosis. It is also a haploid germ cell. It matures into a non-motile spermatozoon via spermiogenesis.
Spermatogenesis vs. Spermiogenesis
Spermatogenesis and spermiogenesis are biological concepts associated with the production of sperm cells. Spermatogenesis, on one hand, is the more-inclusive concept. It includes the processes and events in which spermatogonia give rise to spermatocytes, then to spermatids, and ultimately to sperm cells. Spermiogenesis, on the other hand, is the stage wherein the spermatids differentiate into sperm cells. Spermiogenesis is, therefore, a part of spermatogenesis.
Spermatogenesis is comprised of the following stages: spermatocytogenesis, spermatidogenesis, and spermiogenesis. In the first stage (spermatocytogenesis), there is high mitotic activity. The undifferentiated male germ cells,spermatogonia, undergo mitosis resulting in the production of more spermatogonia. Some of the spermatogonia leave the mitotic phase and enter the second stage, spermatidogenesis. In this stage, the spermatogonia turn into primary spermatocyte that will undergo first meiotic division (or meiosis I). The resulting cells from this division are referred to as secondary spermatocytes. These cells will then proceed to the second meiotic division (or meiosis II) to give rise to haploid germ cells called spermatids. These spermatids enter the final stage, spermiogenesis. In essence, spermiogenesis involves the sequence of events that leads to the formation of spermatozoa (sperm cells). Thus, from a rounded configuration, the spermatids become streamlined. There is no longer any cell division occurring at this stage. Rather, the spermatids transform into spermatozoa. Spermiogenesis is comprised of four major phases, namely the Golgi phase, cap phase, tail phase, and maturation phase. The entire process of spermatogenesis, spermiogenesis included, occurs in the coiled tubules called seminiferous tubules within the testes. After spermiogenesis, the non-motile spermatozoa leave the seminiferous tubule to reach the epididymis where it will become motile and stored for later release. The differences between spermatogenesis and spermiogenesis are shown below.
Table 1: Differences between spermatogenesis and spermiogenesis | ||
|---|---|---|
| Spermatogenesis | Spermiogenesis | |
| Function | Responsible for the formation of mature motile sperm cells, starting from spermatogonia | Responsible for the formation of mature sperm cells that are not yet motile, starting from spermatids |
| Stages/Phases | (1) Spermatocytogenesis (mitotic phase) (2) Spermatidogenesis (meiotic phase) (3) Spermiogenesis and spermiation (differentiation phase) | (1) Golgi phase (2) Cap phase (3) Tail phase (4) Maturation phase |
| End product | Four fully-differentiated motile spermatozoa from each spermatogonium | One mature yet-to-be motile spermatozoon from each spermatid |
| Location | Seminiferous tubules, then epididymis | Seminiferous tubules |
A spermatogenic cycle and wave can be observed along the length of the seminiferous tubule. A spermatogenic cycle is a cycle of progressive stages occurring within a particular segment of the seminiferous tubule. A spermatogenic wave, in turn, refers to the distance from one stage to another similar stage. (1) A single cycle would, therefore, refer to that in which the same stage reappears for a certain duration at a particular segment. (1, 2) The number of stages in a spermatogenic cycle differs among species. Mouse, for example, has a cycle comprised of twelve stages numbered from I to XII occurring over 8.6 days. (2) Humans have six stages (I-VI) in a cycle and it takes four 16-day cycles for a spermatogonium to be transformed into a spermatozoon. (1)
Phases of Spermiogenesis
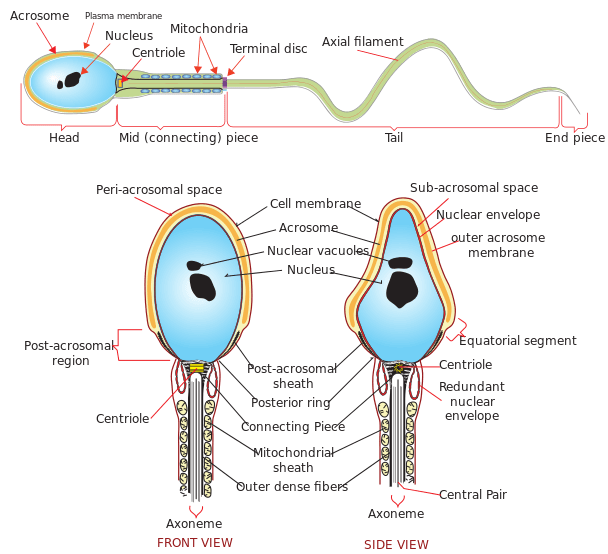
Prior to spermiogenesis, the spermatids are still rounded and large due to the cytoplasmic structures that are still intact. They will go through changes in their form so that they become adapted for fertilization. They will have a distinct, more compacted, smaller, and streamlined head with an anterior acrosome, a thickened mid-piece containing a ring of mitochondria, and a flagellum at the posterior. (1) Spermiogenesis is generally comprised of four major phases: (1) Golgi phase, (2) Cap phase, (3) Tail phase, and (4) Maturation phase.
Phase 1: Golgi phase
The Golgi phase is the initial set of events of spermiogenesis. During this phase, the spermatid is prepared to acquire the major parts of a sperm cell: head, mid-piece, and tail. The reason that the phase is called as such is because of the intensified activity of the Golgi apparatus. During this phase, this organelle produces and releases lytic enzymes that will gather inside the future acrosome (cap phase). At the other end of the spermatid, a mid-piece form. The area thickens as mitochondria fill in this part. Also during this phase, the genetic material (DNA) undergoes packaging as protamines replace histones. (2) By the end of this phase, the genetic material is tightly packed, highly condensed, and transcriptionally inactive.
Phase 2: Cap phase
After the Golgi phase is the cap phase. This phase is characterized by the events leading to the formation of the acrosomal cap (thus, the name). The acrosomal cap or acrosome is a membrane-bound compartment at the tip of the head of the spermatid. It forms when the Golgi apparatus surrounds the anterior of the spermatid to form the acrosome. An acrosome-acroplaxome-manchette complex characterizes this stage. The complex is the major driver for shaping the head of the developing spermatid. (2)
Phase 3: Tail phase
The tail phase is characterized by the elongation of microtubules on one of the centrioles of the spermatid to become the tail. The microtubules form an axoneme. The axoneme contains microtubules that are arranged in a 9 + 2 configuration. This means that a pair of microtubules lies at the center and then surrounded by 9 outer doublet microtubules. (2) The developing germ cell orients itself such that the growing tail is directed toward the center of the lumen of the seminiferous tubule.
Phase 4: Maturation phase
The maturation phase is the final phase. In this phase, the residual cytoplasm has been disposed of. Termed as the residual body of regaud, i.e. the remaining cytoplasm and organelles, is removed by phagocytosis.
The Sertoli cells are epithelial cells of the seminiferous tubules that have several essential roles in spermatogenesis. During the early stages, they nourish the germ cells attached to them. They are also responsible for the secretion of the testis-determining factor, which concentrates testosterone in close proximity. Then in spermiogenesis, these cells are responsible for the phagocytosis of the residual cytoplasm.
The final product of spermiogenesis is a non-motile mature spermatozoon. Thus, the spermatozoa after this stage will still be sterile since they are not yet motile. They become motile cells when they further develop in the epididymis. The migration of the spermatozoon to the epididymis to become a motile sperm cell is called spermiation.
Events Post-spermiogenesis
Spermiation refers to the events after spermiogenesis. The Sertoli cells release the now elongated yet non-motile spermatozoa into the lumen of the seminiferous tubules to be transported to the epididymis where they will become motile spermatozoa. Since the spermatozoa are not motile yet, the Sertoli cells secrete testicular fluid to aid them as they travel from the seminiferous tubules to the rete testis in the mediastinum testis, to the efferent ducts, and finally to the epididymis via peristaltic contraction. The epididymis is the convoluted tubule where they will become motile and be stored for release (ejaculation).
A motile spermatozoon is a mature, fully-differentiated male gamete. It is more commonly known as the sperm cell (or collectively, sperm). From spermatid to a fully functional spermatozoon, the cell acquires morphological transformations that make it adapted to fertilizing an ovum. For instance, the anterior end of a sperm cell is an acrosome filled with enzymes that will assist in breaking down the wall of the ovum (egg cell) so that the sperm can fertilize it. The midpiece contains mitochondria surrounding the axoneme. These mitochondria are the key to the production of chemical energy (ATP) to fuel the flagellar movement. The tail is a flagellum that prompts the cell forward.
Factors
Elevated temperature can cause adverse or detrimental effects on the germ cells in the seminiferous tubules. In humans, for instance, the optimal temperature is at 2 °C lower than the core body temperature. The male body is able to maintain the optimal temperature through blood flow regulation. Moreover, the testes of humans are surrounded by a pouch of skin called the scrotum as they are located externally. This externalized position of the testes is a feature of a certain group of mammals (the boreoeutherians) as opposed to the others whose testes are located internally.
Other factors that might influence the growth and development of germlines include vitamins B, E, and A deficiencies, oxidative stress, and exposure to x-ray, alcohol, anabolic steroids, cadmium, lead, or pesticides.
Lesions and Disturbances
What is currently known about spermiogenesis is that spermatids undergo differentiation and no further cell division occurs. In rats and mice, the period is typically two to three weeks to turn the round spermatids into streamlined spermatozoa. The events of spermiation are genetically and hormonally regulated. Testosterone, for instance, is a major androgenic hormone that incites the expression of nuclear genes associated with the spermiogenic events. Disturbances in these genes could lead to dysfunctional and malformed spermatozoa. Examples of signature lesions indicating a disturbance in this stage of spermatogenesis are misshapen heads, misshapen tails, and multinucleation. (3)
Abnormal acrosome
Disturbances during the acrosome formation could lead to a misshapen head of the spermatozoon. Examples of disturbances may be in the form of microtubule dynamic inhibition or of abnormal deposition of acrosomal granules around the nucleus, especially during the early phase. (3) There are instances when the spermatid fails to form an acrosome, such as in the case of globozoospermia. (3, 4) Globozoospermia is a rare disorder (incidence <0.1%) and one of the causes of male infertility. Individuals with this condition produce spermatozoa devoid of the acrosome.(4)
Abnormal tail
Tail abnormalities of spermatozoa could be due to defects in tubulin or abnormal axoneme assembly. There are instances when the 9+2 axoneme configuration is not achieved, e.g. lacking a pair of central microtubules or missing outer doublets. (3)
Multinucleation
Spermatids, as they grow and go through spermiogenesis, remain connected to each other by intercellular bridges. These connections allow organelles and chromatoid bodies to pass through. ( 3, 5) Thus, the result is multinucleation and a syncytium of spermatids as cytokinesis of meiosis has not been fully completed between the spermatids arising from a single spermatocyte. Not only organelles are shared through these connections but also gene products. Through these bridges, the spermatids could share with the gene products they lack. Take note that the spermatids at this stage are already haploid after the chromosomes separate at meiosis. Therefore, they are genetically dissimilar and may either contain or lack certain genes, e.g. one spermatid contains X chromosome whereas the other spermatid contains Y chromosome. These bridges have also been found to vary in diameter during spermiogenesis. For instance, in rats, the bridge diameter had been found to be 1.8 μm between early step 1 spermatids, and up to 3μm between step 18 spermatids.(3, 6)
Spermatids that are multinucleated might also mean a disturbance. However, it may be caused not just by factors during spermiogenesis but also possibly by a failed function of the Sertoli cells. (3)
These disturbances could lead to the retention of the spermatid at the lumen of the seminiferous tubules. (3)
Biological Importance
Spermiogenesis is an essential biological process as it leads to the transformation of spermatids into mature spermatozoa. Although there are no longer genetic changes and cell divisions occurring at this point, the spermatids undergo morphological changes to prepare them into becoming fully differentiated motile sperm cells. Sertoli cells, the specialized epithelial cells in the seminiferous tubules, help by nourishing them and grooming them into mature spermatozoa. Thus, by the time the cells are liberated from the seminiferous tubules to migrate to the epididymis, they are ready to undergo differentiation into motile sperm cells. Any disruption or interference in the process of spermiogenesis could lead to malformed or dysfunctional spermatozoa. Abnormalities in the head, midpiece, tail, genetic content, and subcellular structures could lead to dysfunctional sperm cells, and thus, to male infertility.
Try to answer the quiz below to check what you have learned so far about spermiogenesis.
See also
References
- “SPERMATOGENESIS.” (2019). Retrieved from Uwyo.edu website: http://www.uwyo.edu/wjm/repro/spermat.htm
- O’Donnell, L. (2014).Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 4(2): e979623.
- Dam, A. H. D. M., Feenstra, I., Westphal, J. R., Ramos, L., van Golde, R. J. T., & Kremer, J. A. M. (2007). Globozoospermia revisited. Human Reproduction Update, 13(1), 63–75. https://doi.org/10.1093/humupd/dml047
- Greenbaum, M. P., Iwamori, T., Buchold, G. M., & Matzuk, M. M. (2011). Germ cell intercellular bridges. Cold Spring Harbor Perspectives in Biology, 3(8), a005850. https://doi.org/10.1101/cshperspect.a005850
- Weber, J. E., & Russell, L. D. (1987). A study of intercellular bridges during spermatogenesis in the rat. The American Journal of Anatomy, 180(1), 1–24. https://doi.org/10.1002/aja.1001800102
© Biology Online. Content provided and moderated by Biology Online Editors