Sugar
n., plural: sugars
[ˈʃʊɡɚ]
Definition: a generic term for any disaccharides and monosaccharides
Table of Contents
Sugar Definition
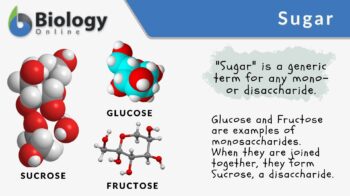
The term sugar is the generic term for any disaccharides and monosaccharides. Sugars are an essential structural component of living cells and a source of energy in many organisms. Sugars are classified based on the number of monomeric units present. The term simple sugars denote the monosaccharides. The term table sugar or granulated sugar actually refers to sucrose, which is a disaccharide made of two monosaccharides: glucose and fructose. Sucrose is a form of sugar that many people are familiar with. It is used in food preparation, such as in cakes, pastries, and desserts. It is also used as an ingredient in several beverages, such as sodas, coffee, and juices.
Sugar is a monosaccharide or a disaccharide. Sugars are mostly known commercially as any sweet crystalline solid disaccharide (sucrose) or monosaccharide (glucose) used as a sweetener or a preservative. Biologically, sugars are used especially by organisms as a source of metabolic energy (ATP). Etymology: from Sanskrit शर्करा (śárkarā), meaning “ground or candied sugar”. See also: carbohydrate, polysaccharide
Carbohydrates
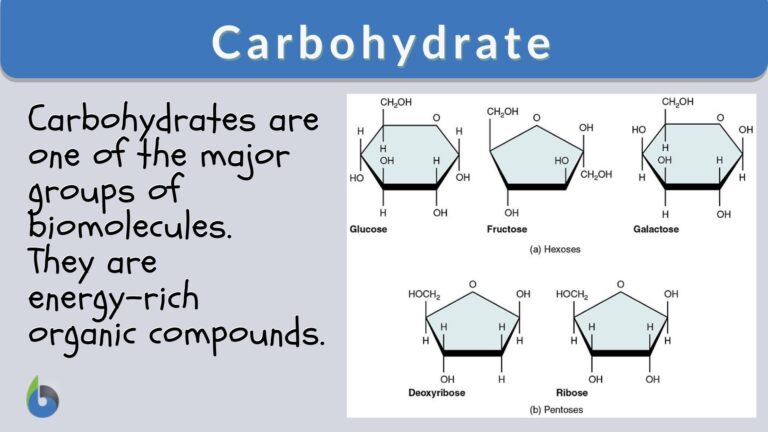
Carbohydrates, especially polysaccharides, are one of the four major groups of biomolecules. The others are proteins, amino acids, and nucleic acids. A carbohydrate refers to any of the group of organic compounds consisting of carbon, hydrogen, and oxygen, usually in the ratio of 1:2:1, hence the general formula: Cn (H2O) n.
Carbohydrates are the most abundant among the major classes of biomolecules. They are one of the major nutrients, providing energy that shall fuel various metabolic processes. As a nutrient, carbohydrates may be classified based on their structural complexity: simple and complex. Simple carbohydrates, sometimes referred to as simply, sugar, are those that are readily digested and serve as a rapid source of energy. Complex carbohydrates (such as cellulose, starch, and glycogen) are those that need more time to be digested and metabolized. They often are high in fiber and unlike simple carbohydrates, they are less likely to cause spikes in blood sugar.
Characteristics of Sugar
Sugars, just like the other carbohydrates, are organic compounds. An organic compound is a compound that, in general, contains carbon covalently bound to other atoms, especially Carbon-Carbon (C-C) and Carbon-Hydrogen (C-H). The four major elements that make up sugars and other carbohydrates are carbon, hydrogen, oxygen, and nitrogen. The general chemical formula of sugar is Cn (H2O) n (or Cn H2nOn), where n may range from 3 to 7. The ratio of hydrogen atoms to oxygen atoms is often 2:1. (NB: An exception to this rule is deoxyribose.) Because of this chemical formula rule, sugars and most carbohydrates are referred to as hydrates of carbon. Most sugars have a name that typically ends in –ose. They may contain either aldehyde or ketone groups.
The saccharide is the structural (monomeric) unit of carbohydrates. Monomers of carbohydrates (i.e. monosaccharides) may join to form longer chains. The monosaccharides are linked to each other (or to other non-carbohydrate groups) by a glycosidic bond (also called glycosidic linkage), a type of covalent bond.
Classification of Sugars
Saccharide is the structural (monomeric) unit of carbohydrates and carbohydrates may be classified into monosaccharides, disaccharides, oligosaccharides, and polysaccharides based on the number of saccharide units.
The most fundamental type is the simple sugars called monosaccharides. Monosaccharides include fructose, galactose, and glucose. Fructose is also called fruit sugar. It naturally occurs in fruits, cane sugar, and honey. It is the sweetest among the sugars. Galactose is another simple sugar but is seen often bound to another molecule. Glucose is the most common form of simple sugar in the body as it is essential in various cellular activities such as cell respiration. In plants, glucose is the primary product of photosynthesis. These monosaccharides are the simplest forms of carbohydrates. They serve as the monomers that join together to form a rather complex carbohydrate, e.g. disaccharides, oligosaccharides, and polysaccharides.
Disaccharides are carbohydrates consisting of two monosaccharides. Examples are lactose, maltose, and sucrose. The table sugar is sucrose, which is a disaccharide made up of glucose and fructose. It is used commonly as a sweetener. It is used in beverages and food preparation, such as cake and cookies. The common sources of sugar for commercial use are sugarcane and sugar beet. These plants are harvested to make refined sugar.
Dietary Sugars
Dietary sources of sugars are mostly from plants, especially sugarcane and sugar beet. Some of the common dietary sources of sugars from fruits are apples, bananas, grapes, oranges, peaches, pears, pineapples, strawberries, and plums. In vegetables, the most common sources include sugarcanes, sugar beets, carrots, yams, and sweet potatoes. Sugarcanes and sugar beets are the two major sources of sugar being sold in the market.
Commercialized sugar is primarily sucrose. Brown sugar is about 97% carbohydrates. It contains molasses, and as such, is light or dark in color and richer in flavor than white sugar. White granulated sugar is 99.9% carbohydrates. It is the common table sugar used as a sweetener at home. Artificial sweeteners are often comprised of synthetic polysaccharide maltodextrin and other sweeteners.
Excessive consumption of sugar is linked to diabetes, obesity, tooth decay, and cardiovascular diseases.
Biological Importance of Sugars
Sugars are important in organisms by serving the following roles or functions:
- Polymer formation
- Structural component
- Source of nutrition and energy for metabolism
- Energy storage
Polymer formation
Simple sugars, particularly monosaccharides, can create natural polymers. Oligosaccharides, for instance, are polymers comprised of up to ten simple sugars. Examples are raffinose, maltotriose, and maltotetraose. Polysaccharides are longer polymers. They are made up of several saccharide units (hence, the name poly). Examples are starch, cellulose, and glycogen.
Structural component
Sugars are an important structural component of various biological materials. For example, nucleic acids, such as RNA and DNA, have a sugar component in them, i.e. ribose and deoxyribose, respectively. Many other biological molecules have sugar components in them, e.g. glycoproteins, glycolipids, proteoglycans, which in turn carry out vital roles, e.g. in immune response, detoxification, blood clotting, fertilization, biological recognition, etc.
Source of nutrition and energy for metabolism
Sugars are a major nutrient. They are one of the major dietary requirements of many living organisms because they provide the body with a source of chemical energy. Simple sugars, since they are in a form easily and readily digestible, provide organisms a compound from where energy fuel can be easily and readily derived. Complex carbohydrates, in contrast, need a longer time to be digested and metabolized.
ATP is chemical energy produced via a series of metabolic processes in cellular respiration.
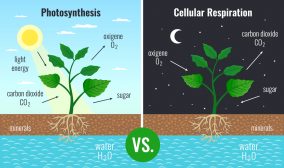
In brevity, glucose (a monosaccharide) is “churned” to extract energy, primarily, in the form of ATP. Firstly, a series of reactions leads to the conversion of glucose to pyruvate. Then, it uses pyruvate, converting it into acetyl coenzyme A for oxidation via an enzyme-driven cyclic reaction called the Krebs cycle. Finally, a cascade of reactions (redox reactions) involving the electron transport chain leads to the production of more ATPs (via chemiosmosis).1
Glucose molecules used in glycolysis are derived from a carbohydrate-containing diet. Complex carbohydrates are broken down into simpler monosaccharides, such as glucose, by digestion.
Energy storage
Monosaccharides, when they are not yet needed, can be stored for later use. They can be converted into energy-rich polysaccharides, particularly, starch in plants and glycogen in animals. In plants, starch is abundant in amyloplasts inside the cells of various plant organs, e.g. fruits, seeds, rhizomes, and tubers. In animals, glycogen is stored in the liver and in the muscle cells.
Common Biological Reactions Involving Sugars
There are various biological reactions that involve sugars. Some of the most common ones are described briefly below.
- Photosynthesis
- Saccharification
- Digestion and Absorption
- Glycolysis
- Gluconeogenesis
- Glycogenesis
- Glycogenolysis
- Pentose Phosphate Pathway
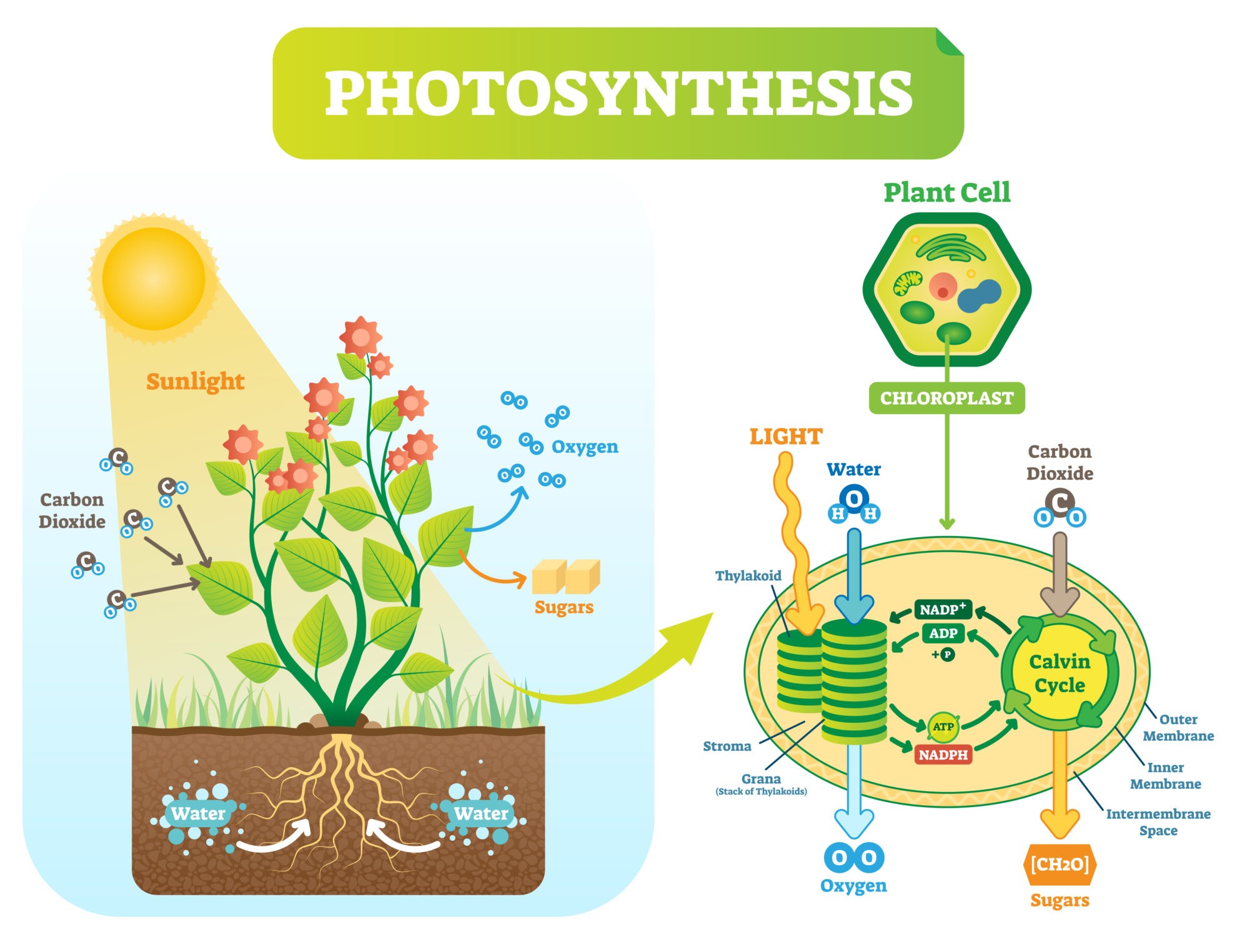
Photosynthesis
Plants and other photoautotrophs produce glucose by means of photosynthesis. Using carbon dioxide, water, inorganic salts, and light energy (from sunlight) captured by light-absorbing pigments, such as chlorophyll and other accessory pigments to produce carbohydrates (e.g. glucose), water, and oxygen molecules.
Saccharification
Monosaccharides form disaccharides and other polymers by joining together via glycosidic bonds. The process is dehydration because the joining of monosaccharides results in the release of water as a byproduct.
The process wherein complex carbohydrates are degraded into simpler forms, such as glucose, is called saccharification. It entails hydrolysis. In humans and other higher animals, this involves enzymatic action. In the mouth, glucose-containing complex carbohydrates are broken down into simpler forms through the action of salivary amylase. In the small intestine, the digestion of complex carbohydrates is continued.
Enzymes such as maltase, lactase, and sucrase break down disaccharides into monosaccharide constituents. Glucosidases are another group of enzymes that catalyze the removal of the terminal glucose from a polysaccharide comprised chiefly of long chains of glucose.
Digestion and Absorption
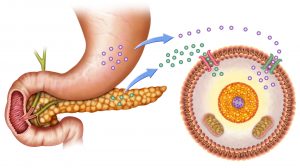
Monosaccharides from the digested carbohydrates are absorbed by the epithelial cells of the small intestine. The cells take them up from the intestinal lumen through the sodium ion-glucose symport system (via glucose transporters or GluT). GluTs are proteins facilitating the transport of monosaccharides, such as glucose, into the cell. Next, they are released into the capillaries by facilitated diffusion. Cells of tissues take them up from the bloodstream again via GluTs.
When inside the cell, glucose is phosphorylated to trap it inside the cell. As an effect, glucose-6-phosphate may be used in any of the following metabolic pathways:
- Glycolysis, to synthesize chemical energy
- Glycogenesis, where glucose is brought to the liver via the vena portae to be stored as cellular glycogen
- Pentose phosphate pathway to form NADPH for lipid synthesis and pentoses for nucleic acid synthesis
Glycolysis
Glycolysis is the initial process of aerobic respiration that occurs in the cytosol. In this metabolic pathway, a series of reactions in the cytosol results in the conversion of a monosaccharide, often glucose, into pyruvate, and the concomitant production of a relatively small amount of high-energy biomolecules, such as ATPs. NADH, an electron-carrying molecule, is also produced. In the presence of oxygen, the process may proceed to the Krebs cycle and oxidative phosphorylation, producing more ATPs. In the absence of oxygen, anaerobic respiration takes place.
Gluconeogenesis
Gluconeogenesis seems like the reverse of glycolysis in a way that glucose is converted into pyruvate whereas, in gluconeogenesis, pyruvate is converted into glucose. Glucose is formed from non-carbohydrate precursors (e.g. pyruvate, lactate, glycerol, glucogenic amino acids). Glucose is formed from hydrolyzing glucose-6-phosphate by the enzyme glucose-6-phosphatase. It is then shuttled from the endoplasmic reticulum into the cytoplasm.
Glycogenesis
Glycogenesis is the metabolic process of producing glycogen from glucose for storage. It takes place mainly in liver and muscle cells. It occurs in response to the high glucose levels in the bloodstream. Exogenous glucose molecules, for instance, are converted into long polymers to be stored inside the cells. When the body requires metabolic energy, glycogen is broken down into glucose subunits through the process of glycogenolysis. Thus, glycogenesis is the opposite process of glycogenolysis.
Glycogenolysis
Glycogenolysis is the process of breaking down stored glycogen in the liver. Doing so produces glucose that could be used in energy metabolism. A single glucose molecule is cut off from the stored glycogen. It is, next, converted into glucose-1-phosphate. The latter, in turn, is transformed into glucose-6-phosphate that can enter glycolysis.
Pentose Phosphate Pathway
It is a glucose metabolic pathway wherein pentoses and NADPH are synthesized in the cytosol. The pentose phosphate pathway serves as an alternative metabolic route in the breakdown of glucose. In animals, it occurs in the liver, adrenal cortex, adipose tissues, testis, etc. This pathway is the main metabolic pathway in neutrophils. Thus, congenital deficiency in the pathway produces sensitivity to infection. In plants, part of the pathway functions in the formation of hexoses from carbon dioxide in photosynthesis.
In this metabolic pathway, fructose, instead of glucose, enters glycolysis. Fructose, though, needs to go through certain steps before it can enter glycolysis. In animals, fructose metabolism occurs in the muscles, the adipose tissues, and the kidney.
Galactose is derived from lactose (milk sugar comprised of a glucose molecule and a galactose molecule). In this metabolic pathway, galactose enters glycolysis by first being phosphorylated via the enzyme galactokinase and then converted into glucose-6-phosphate.
Blood Sugar Level Regulation
There should be appropriate assimilation and catabolism of sugar to ensure proper metabolism. The levels of glucose, for instance, should be regulated and maintained at steady levels. In humans, the regulation of glucose levels in the blood is through the action of hormones, insulin and glucagon. These hormones are produced and released by the pancreatic cells. When the glucose level in the blood is low, the pancreas tends to release glucagon. But when the glucose level in the blood is high, the pancreas releases insulin. This is because glucagon acts by stimulating the production of sugar. It stimulates the conversion of stored glycogen in the liver into glucose that will be released into the bloodstream. Insulin, on the other hand, promotes the uptake of glucose from the bloodstream by the skeletal muscle cells and adipose tissues so that glucose could be converted and stored into glycogen through the process of glycogenolysis.
Try to answer the quiz below to check what you have learned so far about sugar.
Further Reading
References
- Gonzaga, M. V. Mitochondrial DNA – hallmark of psychological stress – Biology Blog & Dictionary Online. (2018, September 29). Retrieved from https://www.biologyonline.com/articles/mitochondrial-dna-hallmark-of-psychological-stress
© Biology Online. Content provided and moderated by Biology Online Editors