Symbiosis
n., plural: symbioses
[sɪmbaɪˈoʊsɪs]
Definition: a close, long-term association between organisms of different species.
Table of Contents
Symbiosis Definition
What is symbiosis? Symbiosis can be defined as any kind of relationship or interaction between two dissimilar organisms, each of which may receive benefits from their partners that they did not have while living alone (Angelard & Bever, 2013).
Previously, the term was restricted to a mutualistic relationship wherein both organisms benefit from the interaction. In mutualism, the relationship between the two organisms depends on each other. Each one gains benefits from the other. This is the type of relationship between different species where both of the organisms in question benefit from the presence of the other. At present, the scope of the term has become broader. Now, the term includes other forms of associations like parasitism and commensalism.
More than a century ago, Anton de Bary proposed the original definition of symbiosis as a long-term relationship between two different species. To complement his original definition, it can be added, mutualism, (beneficial for both), commensalism (beneficial for one, neutral for the other), and parasitism (beneficial for one, costly for the other).
Etymology and history
The term ‘symbiosis’ is derived from the Greek sύν, meaning ‘together’ and βίωsις, meaning ‘living’ and is defined as the persistent association of two or more dissimilar species. Many decades ago, de Bary introduced into German the word “Symbiose” to refer to detrimental as well as beneficial associations of different creatures, and so included forms of parasitism.
A few years later, biologists began to use the term for meaning associations of organisms, from distinct species, that have complementary needs; i.e., for convenient living arrangements in which dissimilar partners live together with some mutual benefit, explicit or implicit (Lewin, 1982).
A large number of symbioses involve a single multicellular eukaryotic organism coupled with one or more microorganisms, including bacteria, viruses, eukaryotic microorganisms, or Archaea. By convention, the larger partner is usually called the ‘host’ and the smaller ones ‘symbionts.’ (Oliver & Russell, 2016).
There are however several barriers in which these symbionts can establish this type of association. Some symbiotic relationships lead to an organelle differentiation, that follows different key processes: recognition by both symbiont and host, engulfment of the symbiont, the failure of defense systems from the host to prevent the symbiosis by a defense reaction, the physiological integration, and finally, genetic integration. These barriers are more clear in endosymbionts, but can also apply to macroorganisms.
There are a lot of cases (some reviewed here), where initiation of morphogenesis is necessary. This includes the differentiation of specialized cells and tissues, which is a clue that an organism has clearly evolved due to long-term symbiont integration. These partners live in symbioses and show unique morphological specializations.
These changes are more obvious when they directly interact for longer periods of time. Some organisms like plants, bacteria, or fungi alternate independently with entirely integrated living (Chapman and Margulis 1998) with their symbionts.
Types of Symbiosis
What is a symbiotic relationship? A symbiotic relationship (definition: any form of a biological relationship between two dissimilar organisms) is an important ecological interaction among various biotic factors in an ecosystem.
What are the 5 types of symbiotic relationships? There are different types of symbiotic relationships and they are characterized essentially by the type and extent of the impact of the association. When both parties are in a mutually beneficial relationship, it is referred to as mutualism. But when only one party is symbiotically benefitting, then it may be construed as a parasitic type of relationship. Let’s find out more about the different types of symbiosis below.
Mutualism
Mutualism is one of the most studied types of symbiotic relationships. It is described as an interaction between individuals from different species that brings in positive (beneficial) effects on each one of the participants. It can affect the reproduction and/or survival of the populations involved. It is a highly dynamic interaction in which there is likely to be continual evolution and coevolution among partner species (Althoff & Segraves, 2016).
Obligate mutualism refers to a type of symbiosis (Holland & Bronstein, 2008). Mutualistic symbiosis involves a close physical association in the long term between participants (Angelard & Bever, 2013). An example of a mutual relationship is lichens. This mutually beneficial relationship consists of algae and a fungus. The fungi bring support and protection while obtaining food from the photosynthetic algae, in which bright colors are light-absorbing pigments (Audesirk et al., 2013). Other examples of mutualisms in the microbial world are the rhizobium-legume symbiosis and arbuscular mycorrhizas.
Commensalism
Commensalism is an interaction where one individual benefits from another species, while the other is unaffected. For example, one organism can provide essential nutrients or resources to another organism. This type of cross-feeding is common in soil organisms (Hartel, 2004).
A good example of this interaction is when one microorganism (a beneficial bacteria) produces an antibiotic against another organism (a pathogen) (Lazarovits et al., 2007) and provides protection to the plant associated with the beneficial bacteria. Some strains of Bacillus and Pseudomonas are known to suppress disease in certain crops (Larkin & Fravel, 1998; Prashar et al., 2013).
An example from the animal kingdom is remoras and sharks. Remoras use a suction disk to attach to its host, which can be a shark, ray, bony fish, sea turtle, as well as other cetaceans or sirenians. Some of the benefits from the association to the remora include transportation, protection from other marine predators, increased courtship/reproduction potential, enhanced metabolic processes, and more feeding opportunities. These remoras are opportunistic symbionts, that feed on parasitic copepods (which constitutes the bulk of their diet), zooplankton and smaller nekton, food scraps from meals of their hosts, and sloughing epidermal tissue and feces of the host (Fertl & Landry, 2018).
Amensalism
Amensalism is the interaction where one species affects another negatively, while the second species has very little if no effect at all on the first (Kitching & Harmsen, 2008). An example of amensalism is the mussel beds and the various infaunal species that it harbors. Mussels are mollusks that can be rich hosts for several species of marine organisms. But, since the substrate (the mussels themselves) is also composed of living organisms, there exists an amensalistic interaction in which those suspension feeders (associated with the mussel beds) affect the mussels negatively, while the mussels have little effect on the feeders. (Dittmann, 1990). Another example is when a plant is shaded out by a taller plant. The shorter plant is adversely affected by the resulting scanty light available for its use in photosynthesis.
Parasitism
The relationships do not have to be mutually beneficial to be considered symbiotic. Around two-thirds of the Earth’s species are believed to be parasites, and parasitism has evolved independently in many different groups (vampire bats, fleas, flatworms, nematodes, several protists, several plants, etc.) (Zeigler, 2014).
Parasitism is an association between different species of organisms (Noble & Noble, 1971) in which the parasite (symbiont) depends on its host to meet its metabolic needs. It involves the uptake of substances that are nutrients to the parasite. They usually live inside or under their hosts, and they generally harm or debilitate them, though do not kill them immediately. Parasites are usually smaller than their hosts and outnumber them.
An example of parasitic relationships is the one of a species of tropical ants (Cephalotes atratus) and a parasitic roundworm that makes its bulbous rear end, called a gaster, look like a juicy red berry (Poinar & Yanoviak, 2008). Other examples are tapeworms (about 1,100 species), as well as fleas (about 2,000 known species) (Zeigler, 2014).
Predation
In the predator symbiotic relationship, one species (the symbiont) kills another species (their host). Unlike parasitism, the symbiont outright kills their host, rather than harming them for a long time. An example of this relationship is the predation on symbiont sea anemones by their host hermit crab (Dardanus pedunculatus) (Imafuku et al., 2000). Some hermit crabs carry sea anemones in their shells, and when these are a period of starvation, they remove their anemones and feed on them.
Examples of Symbiosis
The more we learn about life on Earth, the more we see symbiotic relationships in living organisms. It even seems to have been involved in at least the origins of several diverse, unique, and successful groups such as eukaryotes—where at least mitochondria and chloroplasts are known to be derived from once free-living prokaryotes. Since more than a century ago, there have been proposed and discussed different endosymbiotic theories to explain the origin of eukaryotic cells and their organelles. This theory (known as The Serial Endosymbiont Theory) of eukaryotic cell origins is now well accepted and is one of the most important and dramatic examples of the role of symbiosis in the evolution on Earth. According to these theories, the eukaryotic cell evolved due to the result of endosymbiosis (endosymbiotic unions) between the early free-living prokaryotic (bacterial) cells and other single-celled organisms (Zeigler, 2014). This is a type of reciprocal advantageous association in which one organism lives inside another and it has pivotal importance in symbiogenesis.
Symbioses involve diverse interactions as previously discussed above. These interactions can also be maintained and disrupted if necessary. How the host and the symbiont interact can define the symbiotic relationship. They even have acquired a variety of mechanisms either for maintaining or terminating the symbiosis (Oliver & Russell, 2016).
Let’s take a look at some of the common symbiotic relationship examples found in nature and how their interaction defines their symbiotic relationship.
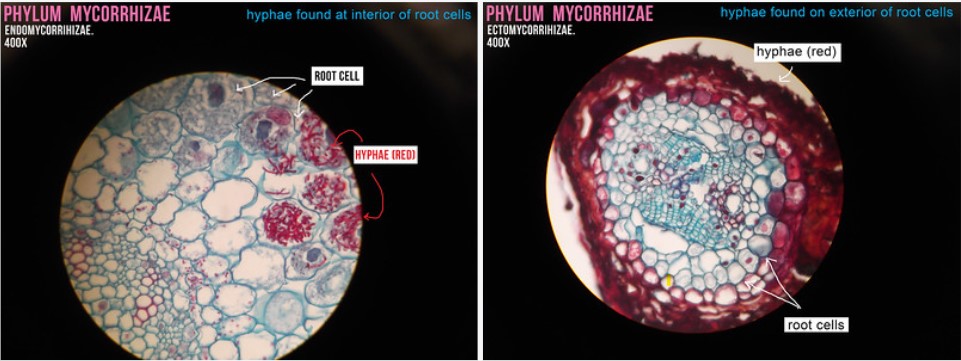
Mycorrhizas
A very particular type of symbiotic interaction is between certain fungi and plants. Fungi symbionts shown in the figure below form arbuscular mycorrhizas (AM), the most common mycorrhizal type. These fungi have been recognized as obligate symbionts of a very wide range of plant species since they are not able to proliferate outside their plant host. The symbioses are biotrophic and normally mutualistic (Schouteden et al., 2015), the long-term compatible interactions being based largely on a two-way nutrient transfer between the symbionts, sometimes supplemented by other benefits such as drought and disease tolerance (Smith & Read, 2008).
Pinworm
Human infection by the parasitic nematode, Enterobius vermicularis, or pinworm, is the most common nematode infection in developed countries and is an example of a parasitic symbiotic relationship. Pinworm infections are commonly found in infants, particularly those who are not toilet trained yet. Adult female worms, which grow up to 1 cm in length, reside in the large intestine. At night, the female worms move out of the host to release eggs on the perianal skin. The eggs become infective within several hours, and the pinworm life cycle is completed when the eggs are ingested and hatch, to release the larval form of the worm in the host small intestine (Jones & Cappello, 2004).
Amebiasis
Parasitic protozoan infections represent a major health problem in developing countries since most contribute significantly to morbidity and mortality. These protozoans can form a symbiotic interaction with their human host that influences their physiology and can make diseases progress slower or faster. In the case of diarrheal diseases, they continue to be major causes of morbidity and mortality in children in developing countries.
In countries like Bangladesh, it is estimated that 1 in 30 children die of diarrhea or dysentery by his or her fifth birthday. Entamoeba histolytica is a protozoan parasite that causes amebic dysentery (Haque et al. 2003). Moreover, there seems to be an influence by other microorganisms living in the host that can affect this symbiosis. Several studies have noted that the bacterial communities in the host can influence the virulence of individual pathogens and potentially add variability to the disease symptoms of parasitic protozoan infections. A study showed that when cultured together with other pathogenic bacteria, Entamoeba histolytica can have their virulent effect augmented or attenuated (Burgess et al. 2017).
Clownfish & anemones
Clownfish (Amphiprion) is one of the most studied ecological symbiotic relationships, and thus an interesting group for the study of symbiosis. Several species can live in close associations with sea anemones (Thalassianthidae, Actinidae, Stichodactilidae). Studied since more than a century ago, this relationship is considered to be a mutualistic symbiosis, as the sea anemones provide protection and nutrients to clownfishes, and clownfishes provide ventilation, nitrogen, and carbon to the host. This is an important role in the nutrition of the anemones that becomes a habitat for clownfish (Roux et al., 2019).
Oxpeckers and different mammals
Yellow-billed oxpeckers (Buphagus africanus) are one of the world’s few obligate mammal gleaners. They exhibit a repertoire of highly divergent adaptations for life with large mammals (like waterbucks or giraffes). These include short, sharp claws that facilitate clinging to hides, as well as long stiff tails that can be used to support the oxpecker when they are clinging onto the bodies of large mammals. This relationship is beneficial to the large mammals too since these oxpeckers have beaks that are laterally flattened and have a sharp cutting edge suitable for handling ticks in their host (Koenig, 1997), which serve as food to the symbiont.
Black walnut tree
A particular type of amensalism interaction is competition. An example of this is the black walnut tree. When it reaches 15-25 years of age, it accumulates allelopathic chemicals that have a detrimental effect on nearby plants, not allowing them to grow. The chemical juglone is responsible for this allelopathic behavior, providing growth inhibition to other competitors for resources (Inderjit & Keating, 1999).

Rhizobium-legume
Since nitrogen is an essential nutrient, plants require it to grow. Even though N2 gas is a major constituent of the atmosphere, it is chemically inert and therefore unavailable as a source of nitrogen for use by most living organisms. However, eukaryotic organisms are unable to fix nitrogen since they do not have the enzymatic machinery to convert inert N2 to usable nitrogen. Because of this, different types of symbiotic relationships have been established between eukaryotes and diazotrophic bacteria that have this function.
Some bacteria can reduce N2 and thereby “fix” atmospheric nitrogen using the enzyme nitrogenase. Many legumes have taken advantage of this special bacterial asset by going into partnership with nitrogen-fixing bacteria, Rhizobium.
In exchange for supplying resources to its bacterial symbiont, the plants receive a supply of reduced nitrogen. Overall, the legumes create a highly specialized environment within their roots, which are suitable for the bacteria to fix nitrogen.
These specialized plant structures are called nodules; usually, they are found on roots, but they also occur on the stems of some legumes (Russo et al., 1992). The symbiotic complex is often referred to as a “symbiosome”.
This structure is known as the basic nitrogen-fixing unit of the nodule. The nitrogen fixed by bacteroids in the symbiosome is exported as ammonium to the host plant cytoplasm, where it is assimilated and transported toward the other organs of the plant. Vice versa, reduced carbon compounds, essential to the bacteria, from the plant are transported to the nodule. Many other metabolites can also be exchanged between the host plant and the symbiosome (Coba de la Peña et al. 2017).
Crabs and sea urchins
Echinococcus pentagonus is a brachyuran crab associated with echinoids throughout the Indo-West Pacific region. In Hawaii, adult females are found inhabiting the rectum of Echinothrix calamaris (Pallas), a diadematidae sea urchin. While males and small females live on the host’s peristome but move freely on its surface, juveniles and adults however depend on the sea urchin for shelter and food. The crab’s nutrition is provided by the host, either by ingestion of tissue or fecal pellets. This crab has been variously categorized as a commensal or a parasite, but it is in fact a symbiont in a long-term relationship (Castro, 1978) since they do not have a major effect on its host.
Human gut microbiota
Microorganisms have a long history of symbiotic relationships with humans. The human microbiota is a complex ecosystem of microbes inhabiting many sites in the human body. The human gastrointestinal (GI) tract is a habitat for a diverse and changing community of microorganisms. They are known as the gut microbiota, and it has been known for quite a long time that they have a marked influence on the host during homeostasis and disease (Thursby & Juge, 2017).
These bacteria have found a suitable ecosystem for their development, and they break down food that humans are not able to digest by themselves, converting them into energy and vitamins (Gut Microbiota for Health; Ley et al., 2008). However, not all microbiota offers a beneficial advantage to their hosts. Each of us eats a distinct diet, containing its own microbiota which can include potential food-borne pathogens. Once ingested, they can be introduced into distinct regions of the gastrointestinal tract, which in turn produce enzymes and induce immune responses that are governed by the host genetics and previous bacterial exposures. There are several cases where foodborne pathogens take advantage of the conditions in their hosts and induce proliferation.
Viruses
While some biologists do not consider viruses as living organisms, they play a key role in their hosts. They can be considered parasites, as they need to complete their viral cycle by infecting cells in order to replicate and infect other individuals. However, there is also controversy in the idea that all viruses are parasitic since some of their associations can also be beneficial. A virus can be commensal if the virus benefits while host’s fitness is unaffected. A virus can also be mutualistic, in which both organisms benefit and fitness increases. These viral associations are not necessarily harmful but might provide advantages that promote the evolution and biodiversity of their hosts (Grasis 2017).
One of the many instances where an organism cannot exist without beneficial viruses is the polydnavirus and wasps. The polydnavirus integration into the parasitoid wasp genetic information, counters the effects of the caterpillar host immune system where the wasp has laid its eggs. Without the viral information encoded in their host, the caterpillar’s immune system would detect the wasp eggs and eliminate them. However, since the polydnavirus endogenous viral element becomes active once the egg is deposited, the host’s immune response to the eggs is hijacked (Herniou et al., 2013).
Watch this vid below for another example of how the virus turned its caterpillar host into a “zombie”, protecting the wasp cocoon that once parasitized the caterpillar.
Table 1: Major animal and plant symbioses. Review by Oliver & Russell (2016)
| Association | Common eukaryotic hosts | Symbiont diversity | Major roles | References |
|---|---|---|---|---|
| Plants – mycorrhizal fungi (MF) | a. Widespread: found in all major plant groups b. Angiosperms/ gymnosperms | a. Arbuscular MF (Glomeromycota) b. Ectomycorrhizal F (mostly Basidiomycota and Ascomycota) | Plants get macronutrients and protection against potential pathogens; fungi get organic carbon; this association is likely responsible for the facilitated colonization of land by plants | (Smith & Read, 2008) |
| Plants – nitrogen fixing bacteria | a. Legumes (Fabaceae) b. Various dicotyledon angiosperms c. Cycads and Gunnera (angiosperms) | a. Rhizobia (α and β-proteobacteria) b. Frankia (actinomycete) c. Cyanobacteria | Plants get usable nitrogen, bacteria get carbon. Can expand host range to nitrogen-poor soils | (Van Der Heijden et al., 2008) |
| Animals – photosynthetic bacteria or algae | a. Corals (Cnidaria) b. Sponges (Porifera) c. Mollusca d. Tunicates (Ascidia) | Symbiodinium (dinoflagellate) (a, b, and c) Chlorella (chlorophyta) (a, b, and c) Cyanobacteria (a and d) | Diverse animals derive carbon fixed from photosynthesis by algae or bacteria. Corals symbioses support communities of marine life | (Venn et al., 2008) |
| Animals – chemosynthetic bacteria | a. Annelid worms b. Bivalve mollusks c. Decapod crustaceans (arthropods) | Sulfur-oxidizing γ-proteobacteria (symbionts that evolved many times). Some ε-proteobacteria and methane oxidizing γ-proteobacteria | Obtaining energy by chemical conversion rather than sunlight-based carbon fixation. This allows primary production of energy and development of ecosystems in deep sea where sunlight does not reach and can not produce photosynthesis. | (Dubilier et al., 2008) |
| Nutrient provisioning, heritable symbionts in insects | a. Sap-feeding Hemiptera b. Blood-feeding hemipterans, lice and flies c. Some ants, roaches, and beetles | Mostly Proteobacteria, but these have evolved many times independently; scarcely fungi | Provides amino acids and cofactors for sap-feeders, B vitamins for blood feeders; symbionts get a stable, nutritional environment. Allowed for niche specialization and subsequent diversification | (Baumann, 2005; Moran et al., 2008) |
| Gut symbionts in animals | Most bilaterian animals harbor gut microbiota | Mostly bacteria ( a diverse taxa), as well as fungi and other eukaryotic microbes | Break down of cellulose and other complex plant polymers, nutrient-provisioning, nitrogen recycling and fixation, detoxify plant defenses and pesticides, protect against ingested pathogens; can also affect development and immune function | (Engel & Moran, 2013; Ley et al., 2008) |
| Facultative, heritable symbionts | Widespread in insects and other arthropods, although infections are not often common within and among host descendants | Mostly bacteria | The host prevents attack from natural enemies, while the symbionts facilitate plant– animal interactions, and even reproductive changes; symbionts also get a stable and nutritional environment | (Moran et al., 2008) |
| Plants – fungal endophytes | a. Grasses b. Most plant species | a. Clavicipitaceous fungal endophytes b. Diverse non-clavicipitaceous endophytes | Fungal produced secondary metabolites that protect plants against herbivores and confer abiotic tolerance; fungi get carbon, protection against predators and growth enhancement | (Rodriguez et al., 2009) |
| Wasp – polydnavirus symbioses | Ichneumonid and braconid parasitic wasps (Insecta: Hymenoptera | Ichnoviruses and Bracoviruses | Protect wasps from insect host’s immune system | (Strand & Burke, 2013) |
| Nematode – bacteria symbioses | Entomopathogenic nematodes (a) Steinernema and (b) Heterorhabditis | a. Xenorhabdus (γ-proteobacteria) b. Photorhabdus (γ-proteobacteria) | Aid in subduing and liquifying insect to aid in resource acquisition from the host | (Bederson et al., 2002) |
| Animals – luminescent bacteria | Most common in marine organisms such as fishes and mollusks like squids and crustaceans | Mostly bacteria including Vibrio and Photobacterium (both γ-proteobacteria) | Used as source of light, also to lure in resources (smaller organisms) for easier acquisition, defense, and interspecific communication | (Stock & Blair, 2008) |
| Agricultural symbioses | Leaf-cutter ants, some termites, and xylophagous beetles | Fungi | Special fungal cultivars gardened for consumption as a food source | (Mueller et al., 2005) |
A new concept has been introduced into symbiosis, which is that of a holobiont. From the examples presented here, it should be clear that symbiotic relationships exist in all living organisms. All individuals live in symbiosis with the microorganisms surrounding them. These symbiotic relationships are necessary for animal health, as a symbiotic breakdown can lead to disease or immunocompromised. The overall physiological symbiosis between the host and their symbionts (which include associated prokaryotes, eukaryotes, and even viruses) in the context of an environment is known as the holobiont (Grasis, 2017).
The concept of “holobiont” was first proposed in 1991 by Lynn Margulis. It was initially meant to refer to a single biological relationship involving one host and a single inherited symbiont. The term was quickly extended to define a host and its associated communities of microorganisms. It is not only limited by the microbiota, but corresponds to the whole collection of microorganisms in interaction with their host. These range from mutualistic, parasitic, and even commensal interactions. Thus, a holobiont is constituted by the host and all of its microbiota. This concept is now widely used in different fields and applies to virtually all organisms, with current research focusing mainly on human, animal, and plant holobionts (Simon et al, 2019). The roles and effects of holobionts have long been studied and described since Anton de Bary introduced the term. What this term intends to update in symbiosis, is the fairly recent realization of the ubiquitous nature of host-associated microbes and their central role in host biology, ecology, and evolution.
Try to answer the quiz below to check what you have learned so far about symbiosis.
References
- Althoff, D. M., & Segraves, K. A. (2016). Mutualism, the Evolutionary Ecology of. In Encyclopedia of Evolutionary Biology (pp. 87–93). Elsevier Inc. https://doi.org/10.1016/B978-0-12-800049-6.00187-6
- Angelard, C., & Bever, J. D. (2013). Symbionts, Genetics of. In Brenner’s Encyclopedia of Genetics (pp. 595–597). Elsevier. https://doi.org/10.1016/B978-0-12-374984-0.01496-0
- Audesirk, T., Audesirk, G., & Byers, B. (2013). Biología La vida en la tierra con fisiología.
- Baumann, P. (2005). BIOLOGY OF BACTERIOCYTE-ASSOCIATED ENDOSYMBIONTS OF PLANT SAP-SUCKING INSECTS. Annual Review of Microbiology, 59(1), 155–189. https://doi.org/10.1146/annurev.micro.59.030804.121041
- Bederson, B. B., Shneiderman, B., & Wattenberg, M. (2002). Ordered and quantum treemaps: Making effective use of 2D space to display hierarchies. ACM Transactions on Graphics, 21(4), 833–854. https://doi.org/10.1145/571647.571649
- Burgess, S. L., Gilchrist, C. A., Lynn, T. C., & Petri, W. A., Jr. (2017). Parasitic Protozoa and Interactions with the Host Intestinal Microbiota. Infection and Immunity, 85(8). https://doi.org/10.1128/IAI.00101-17
- Castro, P. (1978). Settlement and habitat selection in the larvae of Echinoecus pentagonus (A. Milne Edwards), a brachyuran crab symbiotic with sea urchins. Journal of Experimental Marine Biology and Ecology, 34(3), 259–270.
- Chapman, M. J., & Margulis, L. (1998). Morphogenesis by symbiogenesis. International Microbiology: The Official Journal of the Spanish Society for Microbiology, 1(4), 319–326.
- Coba de la Peña, T., Fedorova, E., Pueyo, J. J., & Lucas, M. M. (2017). The Symbiosome: Legume and Rhizobia Co-evolution toward a Nitrogen-Fixing Organelle? Frontiers in Plant Science, 8, 2229.
- Dittmann, S. (1990). Mussel beds – amensalism or amelioration for intertidal fauna? Helgoländer Meeresuntersuchungen, 44(3–4), 335–352. https://doi.org/10.1007/BF02365471
- Dubilier, N., Bergin, C., & Lott, C. (2008). Symbiotic diversity in marine animals: The art of harnessing chemosynthesis. In Nature Reviews Microbiology (Vol. 6, Issue 10, pp. 725–740). Nature Publishing Group. https://doi.org/10.1038/nrmicro1992
- Engel, P., & Moran, N. A. (2013). The gut microbiota of insects – diversity in structure and function. In FEMS Microbiology Reviews (Vol. 37, Issue 5, pp. 699–735). Oxford Academic. https://doi.org/10.1111/1574-6976.12025
- Fertl, D., & Landry, A. M. (2018). Remoras. In Encyclopedia of Marine Mammals (pp. 793–794). Elsevier. https://doi.org/10.1016/b978-0-12-804327-1.00016-9
- Grasis, J. A. (2017). The Intra-Dependence of Viruses and the Holobiont. Frontiers in Immunology, 8, 1501.
- Gut Microbiota for Health. (n.d.). Retrieved September 23, 2020, from https://www.gutmicrobiotaforhealth.com/
- Haque, R., Huston, C. D., Hughes, M., Houpt, E., & Petri, W. A., Jr. (2003). Amebiasis. The New England Journal of Medicine, 348(16), 1565–1573.
- Hartel, P. G. (2004). Microbial Processes – Environmental Factors. In Encyclopedia of Soils in the Environment (Vol. 4, pp. 448–455). Elsevier Inc. https://doi.org/10.1016/B0-12-348530-4/00155-7
- Herniou, E. A., Huguet, E., Thézé, J., Bézier, A., Periquet, G., & Drezen, J.-M. (2013). When parasitic wasps hijacked viruses: genomic and functional evolution of polydnaviruses. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 368(1626), 20130051.
- Holland, J. N., & Bronstein, J. L. (2008). Mutualism. In Encyclopedia of Ecology, Five-Volume Set (pp. 2485–2491). Elsevier Inc. https://doi.org/10.1016/B978-008045405-4.00673-X
- Imafuku, M., Yamamoto, T., & Ohta, M. (2000). Predation on symbiont sea anemones by their host hermit crab Dardanus pedunculatus. Marine and Freshwater Behaviour and Physiology, 33(4), 221–232. https://doi.org/10.1080/10236240009387094
- Inderjit, & Keating, K. I. (1999). Allelopathy: Principles, Procedures, Processes, and Promises for Biological Control. Advances in Agronomy, 67(C), 141–231. https://doi.org/10.1016/S0065-2113(08)60515-5
- Jones, B. F., & Cappello, M. (2004). Nematodes. In Encyclopedia of Gastroenterology (pp. 692–695). Elsevier. https://doi.org/10.1016/B0-12-386860-2/00779-6
- Kitching, R. L., & Harmsen, R. (2008). Amensalism. In Encyclopedia of Ecology, Five-Volume Set (pp. 160–162). Elsevier Inc. https://doi.org/10.1016/B978-008045405-4.00640-6
- Koenig, W. D. (1997). Host preferences and behaviour of oxpeckers: co-existence of similar species in a fragmented landscape. Evolutionary Ecology, 11(1), 91–104.
- Larkin, R. P., & Fravel, D. R. (1998). Efficacy of various fungal and bacterial biocontrol organisms for control of fusarium wilt of tomato. Plant Disease, 82(9), 1022–1028. https://doi.org/10.1094/PDIS.1998.82.9.1022
- Lazarovits, G., Vincent, C., & Goettel, M. S. (2007). Adventures in Biocontrol. In G. Lazarovits, C. Vincent, & M. S. Goettel (Eds.), Biological control: A global perspective (Libro) (pp. 1–6). AAFC.
- Lewin, R. A. (1982). Symbiosis and Parasitism: Definitions and Evaluations. BioScience, 32(4), 254–260. https://doi.org/10.2307/1308530
- Ley, R. E., Lozupone, C. A., Hamady, M., Knight, R., & Gordon, J. I. (2008). Worlds within worlds: Evolution of the vertebrate gut microbiota. Nature Reviews Microbiology, 6(10), 776–788. https://doi.org/10.1038/nrmicro1978
- Moran, N. A., McCutcheon, J. P., & Nakabachi, A. (2008). Genomics and Evolution of Heritable Bacterial Symbionts. Annual Review of Genetics, 42(1), 165–190. https://doi.org/10.1146/annurev.genet.41.110306.130119
- Mueller, U. G., Gerardo, N. M., Aanen, D. K., Six, D. L., & Schultz, T. R. (2005). The Evolution of Agriculture in Insects. Annual Review of Ecology, Evolution, and Systematics, 36(1), 563–595. https://doi.org/10.1146/annurev.ecolsys.36.102003.152626
- Noble, E. R., & Noble, G. A. (1971). Parasitology. The biology of animal parasites. 3rd Edition. Parasitology. The Biology of Animal Parasites. 3rd Edition.
- Oliver, K. M., & Russell, J. A. (2016). Symbiosis, Introduction to. In Encyclopedia of Evolutionary Biology (pp. 282–290). Elsevier Inc. https://doi.org/10.1016/B978-0-12-800049-6.00186-4
- Poinar, G., & Yanoviak, S. P. (2008). Myrmeconema neotropicum n. g., n. sp., a new tetradonematid nematode parasitising South American populations of Cephalotes atratus (Hymenoptera: Formicidae), with the discovery of an apparent parasite-induced host morph. Systematic Parasitology, 69(2), 145–153. https://doi.org/10.1007/s11230-007-9125-3
- Prashar, P., Kapoor, N., & Sachdeva, S. (2013). Isolation and characterization of Bacillus sp with In-vitro antagonistic activity against Fusarium oxysporum from rhizosphere of tomato. Journal of Agricultural Science and Technology, 15(SUPPL), 1501–1512.
- Rodriguez, R. J., White, J. F., Arnold, A. E., & Redman, R. S. (2009). Fungal endophytes: Diversity and functional roles: Tansley review. In New Phytologist (Vol. 182, Issue 2, pp. 314–330). John Wiley & Sons, Ltd. https://doi.org/10.1111/j.1469-8137.2009.02773.x
- Roux, N., Lami, R., Salis, P., Magré, K., Romans, P., Masanet, P., Lecchini, D., & Laudet, V. (2019). Sea anemone and clownfish microbiota diversity and variation during the initial steps of symbiosis. Scientific Reports, 9(1), 1–13. https://doi.org/10.1038/s41598-019-55756-w
- Russo, V. E. A., Brody, S., Cove, D., Ottolenghi, S., Downie, A., & Brewin, N. (1992). The Rhizobium-Legume Symbiosis. In Development (pp. 257–270). Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-642-77043-2_19
- Schouteden, N., Waele, D. De, Panis, B., & Vos, C. M. (2015). Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: A review of the mechanisms involved. In Frontiers in Microbiology (Vol. 6, Issue NOV). Frontiers Research Foundation. https://doi.org/10.3389/fmicb.2015.01280
- Simon, J. C., Marchesi, J. R., Mougel, C., & Selosse, M. A. (2019). Host-microbiota interactions: From holobiont theory to analysis. Microbiome, 7(1), 5. https://doi.org/10.1186/s40168-019-0619-4
- Smith, S. E., & Read, D. (2008). The symbionts forming arbuscular mycorrhizas. In Mycorrhizal Symbiosis (pp. 13–41). Elsevier. https://doi.org/10.1016/b978-012370526-6.50003-9
- Stock, S. P., & Blair, H. G. (2008). Entomopathogenic nematodes and their bacterial symbionts: The inside out of a mutualistic association. Symbiosis, 46(2), 65–75. https://arizona.pure.elsevier.com/en/publications/entomopathogenic-nematodes-and-their-bacterial-symbionts-the-insi
- Strand, M. R., & Burke, G. R. (2013). Polydnavirus-wasp associations: Evolution, genome organization, and function. In Current Opinion in Virology (Vol. 3, Issue 5, pp. 587–594). Elsevier B.V. https://doi.org/10.1016/j.coviro.2013.06.004
- Thursby, E., & Juge, N. (2017). Introduction to the human gut microbiota. In Biochemical Journal (Vol. 474, Issue 11, pp. 1823–1836). Portland Press Ltd. https://doi.org/10.1042/BCJ20160510
- Van Der Heijden, M. G. A., Bardgett, R. D., & Van Straalen, N. M. (2008). The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. In Ecology Letters (Vol. 11, Issue 3, pp. 296–310). John Wiley & Sons, Ltd. https://doi.org/10.1111/j.1461-0248.2007.01139.x
- Venn, A. A., Loram, J. E., & Douglas, A. E. (2008). Photosynthetic symbioses in animals. Journal of Experimental Botany, 59(5), 1069–1080. https://doi.org/10.1093/jxb/erm328
- Zeigler, D. (2014). Symbiosis. In Evolution (pp. 101–110). Elsevier. https://doi.org/10.1016/B978-0-12-800348-0.00012-2
©BiologyOnline.com Content provided and moderated by BiologyOnline Editors.







