Table of Contents
Definition
noun
plural: uridines
u·ri·dine, ˈjʊə.ɹɪ.din
A pyrimidine nucleoside that has uracil attached to the pentose sugar ribose
Details
Overview
A nucleoside is a nucleobase with a five-carbon sugar (either ribose or deoxyribose). It is a glycoside formed from the hydrolysis of nucleic acid. A pyrimidine nucleoside is one in which the nucleobase is a pyrimidine, such as cytosine in cytidine. Cytidine is a nucleoside consisting of cytosine and ribose sugar linked by β-N1-glycosidic bond. When a phosphate group is covalently attached to the sugar, it forms a nucleotide. An example of a nucleotide wherein three phosphate groups are attached to uridine is uridine triphosphate (UTP), one of the building blocks of RNA synthesis.
Characteristics
Uridine is found in all living organisms as a structural component of RNA. The chemical formula is C9H12N2O6. Its molar mass is 244.20 g/mol. Chemically, it is a white, odorless crystalline powder. Nucleosides may be classified into ribonucleosides or deoxyribonucleosides, depending on the sugar component. Uridine is a ribonucleoside based on the presence of a ribose sugar rather than a deoxyribose such as in deoxyuridine (which is a deoxyribonucleoside).
Common biological reactions
Common biological reactions
Nucleosides such as uridine can be produced by de novo synthesis pathways. It is produced often as uridine monophosphate by the decarboxylation of orotidylate catalyzed by orotidylate decarboxylase. Nevertheless, uridine may also be obtained from the diet. When the diet contains nucleotides, the body digests them by nucleotidases to produce nucleosides and phosphates. Nucleosides are degraded into their subcomponents (i.e. nucleobases and sugar) by the action of nucleosidases in the lumen of the digestive tract. Some of the main dietary sources of uridine are RNA-enriched foods such as organ meats.
Biological functions
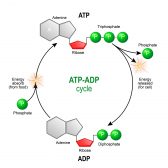
Uridine, just as the other nucleosides, can give rise to nucleotides. When phosphorylated by kinases, the nucleoside is converted into a nucleotide. Thus, a nucleotide is a nucleoside with a phosphate group. Uridine can form uridine monophosphate (UMP, i.e. uridine with a single phosphate group), uridine diphosphate (UDP, i.e. uridine with two phosphate groups), and uridine triphosphate (UTP, i.e. uridine with three phosphate groups). UTP, in particular, is one of the building blocks for the formation of RNA. Apart from nucleic acid synthesis, uridine plays a role in carbohydrate metabolism. In particular, galactose can enter the glycolytic pathway but needs to be converted first into galactose-1-phosphate. It then reacts with UDP-glucose (i.e. UDP attached to glucose) to produce UDP-galactose and glucose-1-phosphate by the catalytic action of galactose-1-phosphate uridyl transferase. The resulting glucose-1-phosphate can then enter the glycolytic pathway.
Supplementary
IUPAC
- 1-(2R,3R,4S,5R)-3, 4-dihydroxy-5-(hydroxymethyl)oxolan-2-ylpyrimidine-2,4-dione
Chemical formula
Synonym(s)
- 1-beta-D-Ribofuranosyluracil
- Uracil riboside
- Uracil-1-beta-d-ribofuranoside
Derived terms
Further reading
See also
- nucleoside
- deoxyuridine
© Biology Online. Content provided and moderated by Biology Online Editors