Valence electron
n., plural: valence electrons
[ˈveɪləns ɪˈlɛk.tɹɑn/]
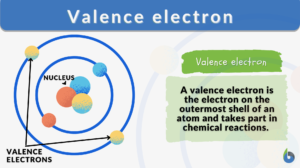
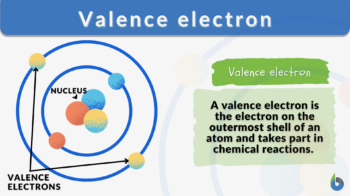
Definition: electrons that are available in the outermost shell of an atom
Table of Contents
What are valence electrons? Why are they significant?
Valence electrons definition in chemistry: The electrons in an atom’s outermost electron shell are called valence electrons. These electrons are the farthest from the nucleus and therefore the least firmly held by the atom. They participate in bonding and chemical processes.
The periodic table can help to understand and determine the valence electrons number an element (particularly a neutral atom of the element) possesses. This also implies that the quantity of valence electrons an element possesses impacts its reactivity, electronegativity, and bonding capacity.
For instance, the valence electron is illustrated in red, which is the simplified picture of sodium’s electrons shown below. It is located on the outside shell (in this case, the shell resembles a ring). (Talk 2022)
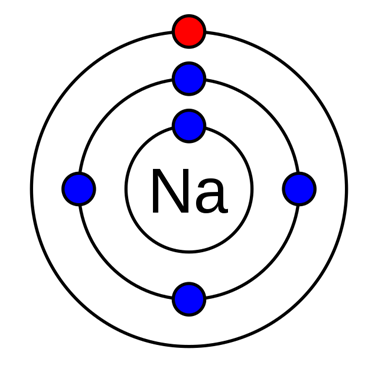
Valence refers to an element’s capacity to establish chemical connections with other atoms. Historically, the valence of an element was determined by the number of hydrogen atoms it could connect to (which is determined by the number of valence electrons it has available for bonding): for instance, carbon can create CH4, thus it has a valence of 4 and four valence electrons. Nitrogen, on the other hand, may form NH3, hence it has a valence of 3 and three valence electrons.
Watch this vid about valency:
Biology definition:
Similar to chemistry and biochemistry, a valence electron is regarded as any electron on the outer shell of the nucleus of an atom. A valence electron is capable of interacting with other atoms, such as when an electron moves to another atom or when electrons are shared. In a single covalent bond, for instance, each of the two atoms shares a valence electron and forms a bond.
Biological importance:
- Molecular interaction: the valence electrons of an atom account for the properties of an element. Based on its valence, it can be determined if the element or compound is capable of bonding with another element and up to what extent.
- Energy absorption/release: A valence electron is capable of absorbing and releasing energy (in the form of a photon).
Valence Electrons and Electronic Configuration
An electronic configuration of an atom refers to the arrangement of electrons in the shells. The highest energy electrons not only determine its valence but also help in determining the chemical behavior of an atom.
Where are electrons located?
The valence electrons of a main-group element are those located in the electronic shell with the largest primary quantum number, n. In this regard, the quantity of valence electrons a molecule may possess is directly significant and also a function of its electron configuration.
Take this for example:
The electronic configuration of phosphorus (P) is 1s2 2s2 2p6 3s2 3p3 (long form). Another way to show its electron configuration is the short form where its core electrons have the same electronic configuration as this: [Ne] 3s2 3p3. This configuration is sometimes expressed in part as Ne for neon (a noble gas), where Ne refers to the core electrons.
However, the energy levels of transition elements are (n-1)d, which are approximately close to the ns energy level. Therefore, a valence electron belonging to a transition element is at a location other than the noble gases core, in contrast to the main group elements. Even though they are not in the outermost shell, d electrons in transition metals act as valence electrons in general.
1s2 2s2 2p6 3s2 3p6 3d5 4s2 represent the electronic configuration of Manganese (Mn), which can also be written as (Ar) 3d5 4s2, where the electronic configuration of (Ar) is similar to the argon which is a noble gas. In these atoms, the 3d and 4s electrons have the same energy levels and significantly have more energy levels than 3s or 3p electrons. There are seven valence electrons present outside the core of argon [Ar] 3d5 4s2. This agrees with the scientific fact that manganese might reach an oxidation state of +7.
How Many Valence Electrons Does an Element Have?
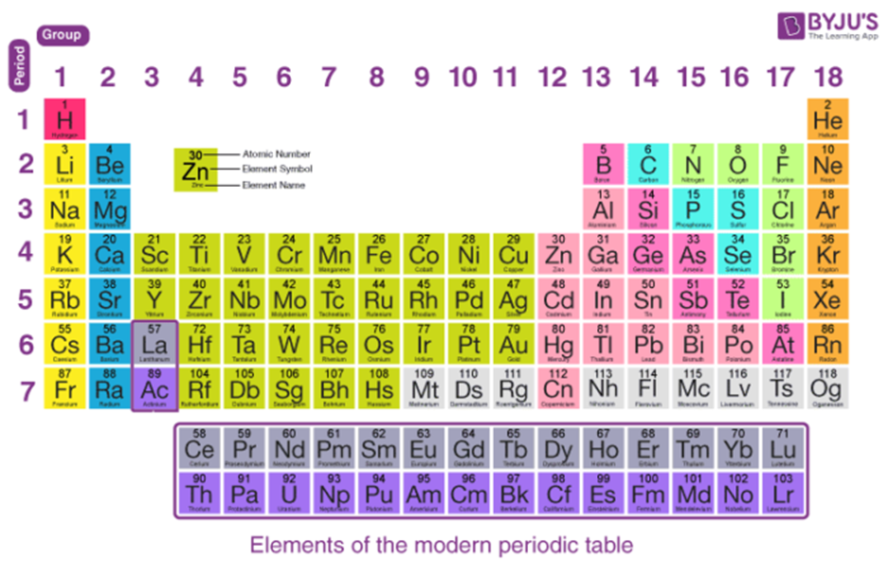
The periodic table can help to understand and determine the valence electrons number an element (particularly neutral atoms of the element) possesses. Consider the element’s group, as the group number shows the number of valence electrons the element possesses.
Notably, this restriction applies specifically to elements that are not transition metals. Transition metals have more complex electron structures. To determine this, it is necessary to examine the element’s unique electron shell configuration.
This also implies that transition metals should be excluded when examining group numbers. They are situated in the middle block of the periodic table. In this view, groups 1 and 2 in the figure below remain the same, while group 13 becomes “group 3,” group 14 becomes “group 4,” and so forth.

Determination of Valence Electrons
Now let us know how to determine valence electrons.
Checking the position of elements in the periodic table is one of the more basic approaches to locating its valence electrons. A thorough investigation of the vertical column in which the elements are arranged will reveal the element’s valence electrons.
This can be accomplished by looking at the column from the side. We can determine the valence electrons number that an element is made up of by looking at the group number that is provided and comparing it to the total number of electrons in that column.
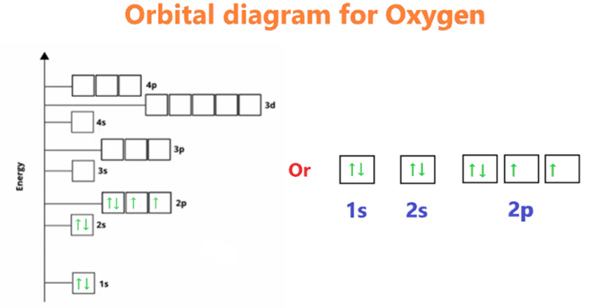
Knowing the electrical configuration is another method that can be utilized for locating or determining valence electrons.
However, locating the valence electron in transition metals (groups 3–12) is a difficult job due to the complexity of the scenario. The atomic structure of these elements is rigid, and the d subshell is missing valence electrons, which causes it to lie below the outer shell.
Valence Shell
What is the valence shell?
The set of orbitals that are capable, from an energy point of view, of accepting electrons to produce chemical bonds is referred to as the valence shell.
The ns and np orbitals are found in the outermost shell of an electron that makes up the valence shell for main-group elements. There are the incomplete orbitals for transition metals, as well as the incomplete subshells for lanthanides and actinides.
There is a possibility that the involved orbitals are located in the inner shell of electrons. Furthermore, the involved orbitals are not at the same electron shell or main quantum number n, they all are present at comparable distances from the nucleus.
In most cases, a reaction between two main-group elements (other than hydrogen and helium) will result in the formation of s2p6 electron configurations. Each linked atom has a total of eight electrons, which also includes the shared electrons, this is called the octet rule.
In the same manner, we achieve the formation of d10s2p6 electron configuration as a result of a reaction with a transition metal. This observed behavior is called the “18-electron rule”, every linked atom has a total of 18 valence electrons, shared electrons included.
Take note though that atoms are most stable when they have a filled valence shell of electrons.
Chemical Reactions
The strength of an atom’s bonds is directly proportional to the number of its n valence electrons.
The group I alkali metals, like sodium (Na) and potassium (K), is one of the types of metallic elements which show high reactivity. This is because there would be just one valence electron in such an atom. In the process of formation of ionic connection, the one extra valence electron can lose easily. During this, ionization energy is obtained. This results in the development of a cation (positive ion) that has a closed shell. To create a closed-shell positive ion (like Mg2+), each atom must lose two of its valence electrons. So, the alkaline earth metals of group 2, like Mg, become less reactive than other metals in the same family.
Within every group (column) of metals comprising the periodic table, reactivity increases while moving towards the lower row, i.e. from a lighter element to a heavier one. The reason is that moving from top to bottom the electron shell is added in every row and their valence electrons have greater primary quantum numbers than those of lighter elements.
In non-metals, the valence electrons are more attractive by atom to full valence shell; this can be done in 2 different ways:
- Either an atom makes a covalent bond by sharing its electron with the neighboring atoms
- Or, forms an ionic bond by gaining or losing an electron
The nonmetal element with the highest reactivity is a halogen, such as fluorine or chlorine.
In the formation of an ionic bond, the electrons are removed from other atoms by a halogen atom (e.g., Cl–, F– etc.). In a covalent bond, one of the halogen’s electrons and one of the other element’s electrons must couple up to establish a covalent bond (e.g., in the case of molecule H-F, one electron shared by H2 and seven electrons shared by fluorine, represent this shared pair of valence electrons).
In moving from top to bottom (light to heavy elements) in the non-metals group, the reactivity is decreased. This happens because the valence electrons have progressively greater energies and are, as a result, less firmly bonded.
In reality, the 16 group has the lightest element O2. It is the second most reactive element after fluorine in the non-metals group, despite not being a halogen. This is due to the fact that the valence shell of halogens has a larger main quantum number.
In Biology (Relevance of term in biology)
The valence electron has the ability to accept and release energy (in the form of a photon). The quantum unit of light energy or electromagnetic radiation is the photon.
As electrons transition from one energy state to another, photons are emitted (when electron loses energy), and when emitted by a biological system, they are referred to as biophotons. This occurs in species with bioluminescence capabilities. In photosynthesis, photons that are absorbed by the chlorophyll’s reaction centers (e.g. P680) trigger the latter to become an electron donor.
FAQs
How to find valence electrons??
One of the simplest methods to identify valence electrons in the periodic table is by checking out the elements’ location in the table. Close inspection of the vertical column in which the elements are arranged reveals information on the valence electrons of each element. Group numbers tell us how many valence electrons each element in a particular column has, thus we can use them to sort elements by their electronic properties. As an illustration, carbon resides in group 4 and possesses a total of 4 valence electrons. Oxygen is a member of group 6 and possesses a total of six valence electrons.
What is valence electron, with example?
The total number of electrons that are present in the orbit of the valence shell is referred to as the valence electron count. Take for instance: since there are six electrons in the last orbital shell of oxygen, the number six is the valence electron.
What is the purpose of valence electrons?
The electrons that are available in the outermost shell of an atom is called valence electron. The reactivity of an atom can be primarily related to its valence electrons.
How do valence electrons work?
Those electrons that are available in the outermost shell of an atom are called valence electrons. Since the electrons in an atom’s outermost shells are the first ones to come into contact with each other during an interaction between two atoms, these electrons are the ones responsible for determining how an atom will behave during a chemical reaction.
Why are valence electrons important?
Valence electrons are important as they help in determining how atoms interact with other atoms and molecules.
Important Notes: Q&As | |
|---|---|
| Question | Answer |
| Where are valence electrons located? | In the outermost shell of an atom |
| How many valence electrons does hydrogen have (H valence electrons)? | 2 |
| How many valence electrons does fluorine have (F valence electrons) (fluorine valence electrons)? | 7 |
| How many valence electrons does sodium have (Na valence electrons)? | 1 |
| How many valence electrons does carbon have (C valence electrons)? | 4 |
| How many valence electrons does nitrogen have? | 5 |
| How many valence electrons does aluminum have? | 3 |
| How many valence electrons are in chlorine (Cl valence electrons)? | 7 |
| How many valence electrons does oxygen have (O valence electrons)? | 6 |
| How many valence electrons does sulfur have (S valence electrons)? | 6 |
| How many valence electrons does potassium have? | 1 |
| How many valence electrons does magnesium have? | 2 |
| Ca valence electrons? | 2 |
| Li valence electrons? | 1 |
| Be valence electrons? | 2 |
| Argon valence electrons? | 8 |
| Phosphorus valence electrons? | 5 |
| I valence electrons? | 7 |
| Calcium valence electrons? | 2 |
| Se valence electrons? | 6 |
| He valence electrons? | 2 |
| Co valence electrons? | 10 |
| Te valence electrons? | 6 |
| Neon valence electrons? | 8 |
| Bromine valence electrons? | 7 |
| What is the valence electron of beryllium? | [He] 2s² |
| What does vsepr stand for? | valence shell electron pair repulsion |
| O2– electron configuration? | [He] 2s² 2p⁴ |
Data Source: Shoaib Zaheer of Biology Online
Answer the quiz below to check what you have learned so far about valence electrons.
References
- BYJU’S (2022). “Valence Electrons.” Retrieved 11 July, 2022, from https://byjus.com/chemistry/valence-electrons/.
- ChemistryTalk. (2022). “valence electrons.” Retrieved 11 July, 2022, from https://chemistrytalk.org/what-are-valence-electrons/.
- TheImportantSite (2022). “Valence electrons determine how atoms interact with other atoms or molecules.” Retrieved 11 July, 2022, from https://theimportantsite.com/why-valence-electrons-are-important/#:~:text=Valence%20electrons%20determine%20how%20atoms,with%20other%20atoms%20or%20molecules.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.