van’t Hoff’s law
In stereochemistry, all optically active substances have one or more multivalent atoms united to four different atoms or radicals so as to form in space an unsymmetrical arrangement, the osmotic pressure exerted by any substance in very dilute solution is the same that it would exert if present as gas in the same volume as that of the solution; or, at constant temperature, the osmotic pressure of dilute solutions is proportional to the concentration (number of molecules) of the dissolved substance; i.e., the osmotic pressure, π, in dilute solutions is π = RTσci, where R is the universal gas constant, T is the absolute temperature, and ci is the molar concentration of solute i, the rate of chemical reactions increases between two-and three-fold for each 10?C rise in temperature.
Dictionary > Vant hoffs law
You will also like...

Darwin and Natural Selection
This tutorial investigates the genetic diversity in more detail. It also delineates how certain alleles are favored over..

Insects
There are more species of insects than any other species combined. This surely illustrates that insects have the selecti..

Mammalian Ancestors
Mammals are a diverse group of organisms, where most of them develop their offspring within the uterus of the mother. Ov..

Neural Control Mechanisms
Neurons generate electric signals that they pass along to the other neurons or target tissues. In this tutorial, you wil..
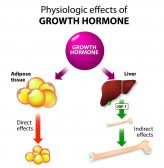
Animal Growth Hormones
Hormones are produced in the endocrine glands of animals. The pituitary gland and hypothalamus are the most impor..
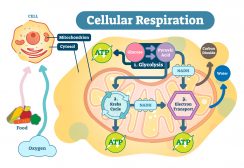
Cell Respiration
Cell respiration is the process of creating ATP. It is "respiration" because it utilizes oxygen. Know the different stag..

