van’t Hoff’s law
In stereochemistry, all optically active substances have one or more multivalent atoms united to four different atoms or radicals so as to form in space an unsymmetrical arrangement, the osmotic pressure exerted by any substance in very dilute solution is the same that it would exert if present as gas in the same volume as that of the solution; or, at constant temperature, the osmotic pressure of dilute solutions is proportional to the concentration (number of molecules) of the dissolved substance; i.e., the osmotic pressure, π, in dilute solutions is π = RTσci, where R is the universal gas constant, T is the absolute temperature, and ci is the molar concentration of solute i, the rate of chemical reactions increases between two-and three-fold for each 10?C rise in temperature.
Dictionary > Vant hoffs law
You will also like...
Human Reproduction and Fertilization
For human species to obviate extinction, reproductive mature adults should be producing viable offspring in order to con..

Plant Tissues
Plant organs are comprised of tissues working together for a common function. The different types of plant tissues are m..

Evolution of Life – Ancient Earth
Autotrophs flourished, absorbing carbon and light. Soon after, primitive life forms that could assimilate oxygen thrived..
Circulation
The circulatory system is key to the transport of vital biomolecules and nutrients throughout the body. Learn about the ..
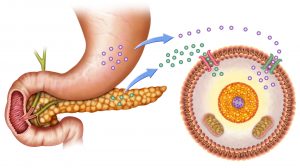
Sugar Homeostasis
The blood sugar level is regulated by two hormones. The mechanism behind this type of negative feedback control is descr..
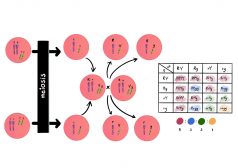
Independent Assortment and Crossing Over
This tutorial describes the independent assortment of chromosomes and crossing over as important events in meiosis. Read..

