Vmax
n.
Definition: The maximum initial velocity or rate of a reaction
Table of Contents
Vmax Definition
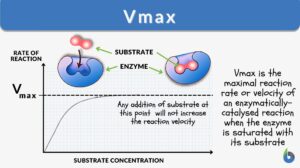
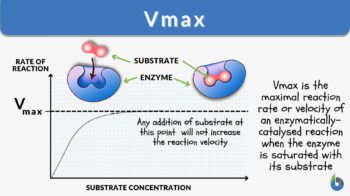
Vmax is the maximal reaction rate or velocity of an enzymatically catalyzed reaction when the enzyme is saturated with its substrate. At a specified enzymatic concentration, temperature & pH, this maximal rate of reaction is the characteristic feature of a particular enzyme. The Vmax unit is moles/min, moles/sec, µmoles/min, or µmoles/sec. Vmax depends upon the amount or the concentration of the enzyme as well as the structure of the enzyme.
Vmax pharmacology, Vmax chemistry, Vmax biochemistry, Vmax kinetics, and Vmax enzyme imply the same. They define Vmax as the maximum velocity or rate of reaction when all enzymatic binding sites are fully occupied or saturated. The enzymes are the biological catalyst that hastens biochemical reactions in a biological system. A typical biochemical reaction involves a substrate (S) and enzyme (E) that results in the formation of a product (P):
S ————————————–> P
E
Biology definition:
Vmax is the maximum initial velocity or rate of a reaction. In enzyme kinetics, Vmax is the maximum velocity of an enzymatically catalyzed reaction when the enzyme is saturated with its substrate. Since the maximum velocity is described to be directly proportional to the enzyme concentration, it can therefore be used to estimate enzyme concentration. Vmax is one of the enzyme kinetic constants that are used to describe the biochemical reaction.
Now let us understand how to calculate Vmax Or how to find Vmax …
Vmax and other enzyme kinetic constants like Km are determined graphically. For the estimation of Vmax, the amount or concentration of enzymes, temperature, and pH are kept constant; only the concentration or amount of substrate is varied.
Let’s understand this with the help of a simple experiment.
To estimate the enzyme’s Vmax E, different concentrations/dilutions of the substrate (S) are taken in the test tubes, namely, 0 M, 2M, 4M, 6M, 8M, 10M, and 12M. A fixed amount of E is added to each test tube and the rate of reaction for each test tube is determined.
A graphical plot between the rate of reaction and substrate concentration is then plotted. This graph is characteristically hyperbolic in shape for an enzymatically-catalyzed reaction (Figure 1). The rate of reaction is the amount of product formed in a unit time. Initially, when the amount of substrate is low, there would be a fast response between substrate and enzyme. The whole substrate would be consumed at lower substrate concentrations. This initial reaction rate is known as the initial velocity (V0). V0 is rapid and is effectively linear.
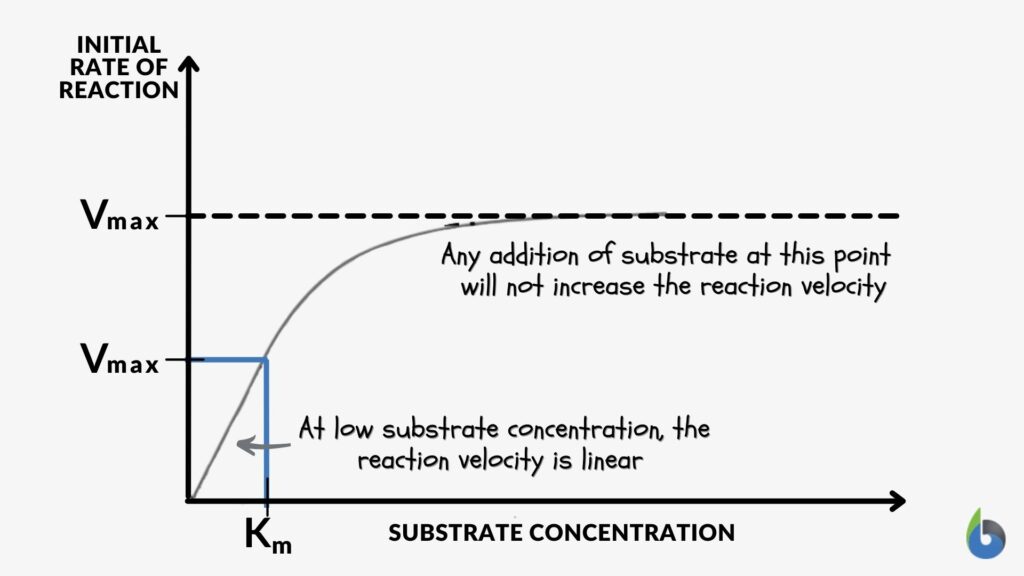
As the concentration of substrate increases, a state of enzyme saturation will reach wherein the substrate occupies the whole enzyme. Beyond this substrate concentration, any increase in its amount would not alter the reaction rate resulting in a plateau phase in the enzyme kinetics graph (Figure 1). The rate of reaction at this stage is the maximum velocity of the enzymatic reaction known as Vmax.
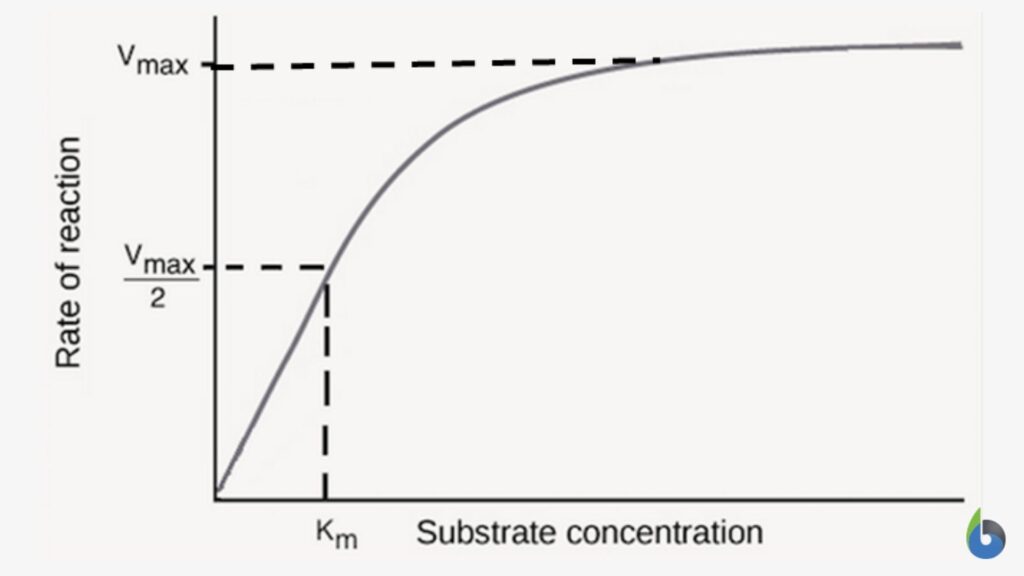
The substrate concentration at which half of the maximum velocity is reached, i.e., Vmax /2, is known as Km or Michaelis-Menten constant. Km is the enzyme kinetic constant that measures the affinity of the enzyme towards the substrate. There is an inverse relation between Km and affinity between enzyme and substrate. A lower Km value indicates a higher enzyme affinity towards the substrate. In comparison, a higher Km value indicates lower enzymatic affinity towards substrate (Figure 2).
The relationship between Km and Vmax can be described with the help of the Michaelis-Menten equation. Vmax formula is-
V=Vmax [S] / Km + [S]
Where,
V= velocity or rate of reaction
S= concentration of substrate
Vmax= Maximum velocity at a saturated concentration
Km= Michaelis-Menten constant
Vmax depends upon the enzyme concentration whereas Km is the enzyme kinetics constant independent of the enzyme concentration. Thus, Km remains unaffected by the change in the enzyme concentration.
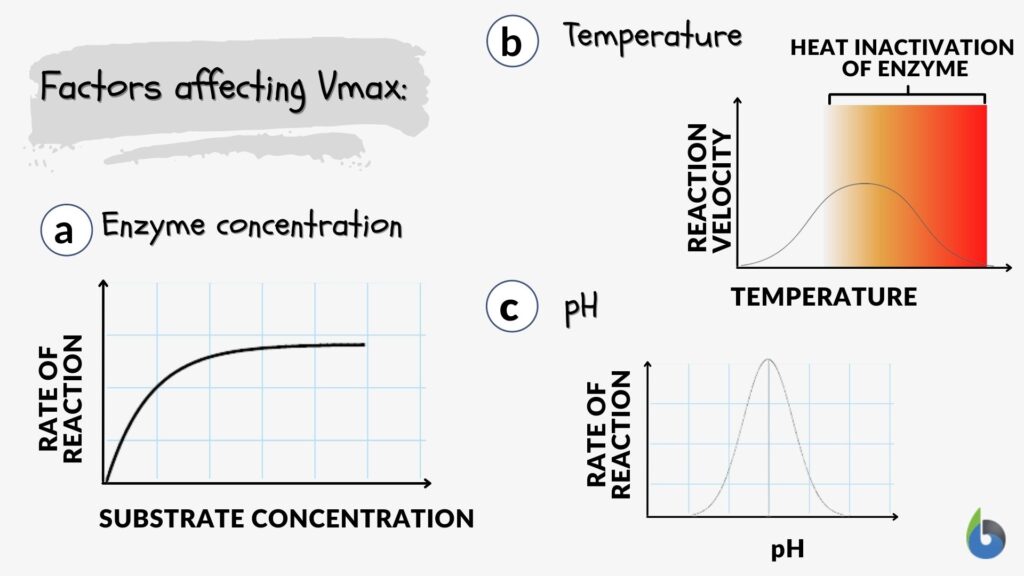
Factors Affecting Vmax
Let us understand what affects Vmax. Vmax value is influenced by three main factors, namely, enzyme concentration, temperature, and pH.
Enzyme concentration
At a constant enzymatic concentration, reaction rate kinetics exhibit a typical hyperbolic curve (Figure 3a) wherein, initially, when the amount of substrate is less, the reaction is fast. However, as the concentration of substrate increases, the enzyme becomes saturated and an increase in the amount of the substrate has no effect on the rate of reaction, i.e., plateau phase. Thus, the amount of enzyme becomes the rate-controlling parameter, and an increase in the enzymes increases the maximal velocity or Vmax. Therefore, the higher the enzyme amount, the higher the Vmax of the reaction. This clarifies the expected query, does Vmax change with enzyme concentration?
Temperature
The higher the temperature, the higher the rate of reaction or the velocity of the reaction. However, once the Vmax is reached, the reaction rate decreases with the increase in temperature (Figure 3b). Usually, beyond Vmax, an increase in temperature results in the denaturation of the enzymes. Most human enzymes get denatured above 40ºC. The optimum enzymatic temperature for most of the human enzymes is around 37ºC.
pH
Different enzymes have different optimum pH requirements, e.g., enzymes present in the stomach (pepsin) need the acidic environment for optimum functioning. In contrast, intestinal enzymes need an alkaline environment. Still, most enzymes get denatured on exposure to extreme pH conditions (Figure 3c).
Significance of Vmax
Vmax and Km are the enzyme kinetic constants used to describe and predict the kinetic behavior of the enzymatically-catalyzed biochemical reaction as a function of the substrate.
- Estimating the Vmax and Km value for two enzymes that work with different pathways on the same substrate can help predict the preferred enzymatic pathway.
- Vmax depends on the amount of the enzyme present; hence, the higher the amount of enzyme, the higher the Vmax of it. Therefore, for comparing the same enzyme from two different sources, the amount of enzyme must be kept the same.
- Km is a good predictor of the effect of the amount of substrate on the formation of the product. An enzyme with a low Km value will remain saturated irrespective of the amount of substrate present in the reaction. On the contrary, an enzyme with high Km will remain unsaturated, and the amount of substrate will highly influence the formation of the product.
- Vmax is also helpful in calculating turn over number (Kcat) of the enzyme, as below:
Kcat =Vmax / [Enzyme]
Kcat is independent of the amount of the enzyme present.
Vmax Examples
Examples of Vmax of some of the common enzymes are as below:
Table 1: Vmax and Km of some of the common enzymes | |||
|---|---|---|---|
| Enzyme | Vmax | Km | Turnover number (per sec) |
| Chymotrypsin | 100 | 5000 | 100 |
| Carbonic Anhydrase | 600,000 | 8000 | 600,000 |
| Penicillinase | 2000 | 50 | 2000 |
| Lysozyme | 0.5 | 6 | 0.5 |
Conclusion
Enzymes are widely used for industrial purposes as biochemical catalysts, therapeutically, analytical reagents, and biotechnological tools. Hence, understanding enzyme reaction kinetics becomes critical. Vmax and Km are useful enzyme kinetics constant that helps understand the feasibility, spontaneity, and fate of the enzymatic reaction. Having a basic understanding of the reaction kinetics further helps control or manipulate the reaction for a higher yield and efficient reaction process.
Try to answer the quiz below to check what you have learned so far about Vmax.
References
- Adrio, J. L., & Demain, A. L. (2014). Microbial enzymes: tools for biotechnological processes. Biomolecules, 4(1), 117–139. https://doi.org/10.3390/biom4010117
- Counotte, G. H., & Prins, R. A. (1979). Calculation of k and v from substrate concentration versus time plot. Applied and environmental microbiology, 38(4), 758–760. https://doi.org/10.1128/aem.38.4.758-760.1979
- Robinson P. K. (2015). Enzymes: principles and biotechnological applications. Essays in biochemistry, 59, 1–41. https://doi.org/10.1042/bse0590001
- Silverstein T. P. (2019). When both Km and Vmax are altered, Is the enzyme inhibited or activated?. Biochemistry and molecular biology education : a bimonthly publication of the International Union of Biochemistry and Molecular Biology, 47(4), 446–449. https://doi.org/10.1002/bmb.21235
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.