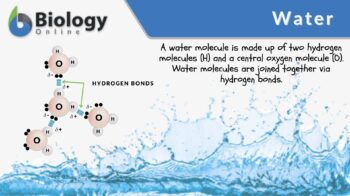
Definition: a molecule consisting of two hydrogen atoms bound to the central oxygen atom via a covalent bond
Table of Contents
Water Definition
(1) (biochemistry) A chemical substance, with chemical formula H2O, that is a clear, colorless, odorless, and tasteless liquid that may also occur in various forms such as gas (water vapor) and solid (ice)
(2) An aqueous solution of a substance, for example, ammonia water, wastewater
(3) A body of water, like seas, rivers, and lakes, and naturally-occurring water like mineral water
(4) Amniotic fluid, as in the pregnant woman’s water breaks
Water (Chemical Formula: H2O) refers to a chemical substance consisting of two hydrogen atoms attached to the central oxygen atom via a covalent bond. This configuration results in a molecule that is polar. Since a water molecule is polarized, the electropositive hydrogen of one water molecule is electrostatically attracted to the electronegative oxygen atom of the nearby water molecule. The electrostatic dipole-dipole interaction between water molecules is called hydrogen bond.
The transient hydrogen bonds between water molecules form a transparent, colorless, odorless, and tasteless liquid. Apart from liquid, water may also occur in other forms such as gas (as water vapor) and solid (as ice). The hydrogen bonds between water molecules are responsible for the water’s distinctive properties, such as high boiling point (100 °C), high surface tension, specific heat, and heat of vaporization.1
Water Properties
Water is regarded as the universal solvent primarily due to its chemical and physical properties. It is one of the major inorganic compounds of biological importance; the others are carbon dioxide, oxygen, and mineral substances and salts. Some of the distinctive properties of water are listed below.
Water is a liquid under standard conditions. It is a tasteless and almost odorless liquid at ambient temperature and pressure. It is also transparent, making it possible for aquatic plants to live in since sunlight can penetrate through. Water, when solid, is referred to as ice. Water expands (rather than shrink) at a temperature less than 4 °C. Thus, it becomes less dense upon freezing. This causes the solidified water (ice) to float above the surface. Consequently, organisms can still manage to live down below the icy surface of oceans and seas. Water has a high heat of vaporization and its gaseous state is called water vapor. The water vapor on Earth is carried by convection into the atmosphere. This leads to the formation of clouds. The clouds, in turn, provide rainwater to living organisms.
A polar molecule
A water molecule is comprised of one centrally-located oxygen and two relatively smaller hydrogen atoms bound to it. Each of these hydrogen atoms is attached to the oxygen by a covalent bond. This results in a partially positive pole and a partially negative pole (thus, making water a polar molecule). Apart from the covalent bond, it forms a transient hydrogen bond with a nearby water molecule. Water molecules stick to one another by a hydrogen bond.
Water is a polar molecule due to its oxygen that has a slight negative charge while its hydrogens have a slight positive charge. Its polarity makes it an excellent solvent for many substances. The slightly negative oxygen attracts cations whereas the slightly positive hydrogen attracts anions. Thus, water has the ability to dissociate and ionize molecules.
High surface tension
Water has relatively high surface tension. This means that the water molecules have a greater attraction with each other (due to cohesion) than with the molecules in the air (due to adhesion). Water has low viscosity, which means that it has low resistance to flow. These properties are essential to plants by slowing down water loss from the leaf stomata. Insects that can walk on water are aided to do so via the high surface tension of water.
Adhesion properties
Water has high adhesion properties. Because of this, water has the ability for capillary action, the tendency to move up a narrow tube against gravity, a property relied upon by vascular plants, such as trees.
Specific heat properties
Water has the second highest specific heat capacity (after ammonia). Specific heat refers to the amount of energy (in calories or joules) required to raise the temperature of one gram of a pure substance by 1 degree Celsius. The high specific heat of the water helps keep the temperature on Earth suitable for life. For instance, the ocean absorbs much of solar radiation during the day and then dissipates it into the atmosphere at night.
Biological Importance
Water is one of the substances essential to life. Biomolecules (e.g. DNA, proteins, and polysaccharides), gases, vitamins, etc. are dissolved in water. The water may act as a transport medium to convey these substances to various parts of the body. It also acts as an important reactant in certain biochemical processes.
In plants, water is an essential requirement in photosynthesis. Since water molecules are polar, they can form transient hydrogen bonds between them and thereby help in the formation of biomolecular structures such as DNA and proteins. It is also because of the water’s polarity that it can interact with ions and other polar molecules.
Water is also important in dissociating compounds into ions. Thus, it helps regulate pH levels. Molecules or substances that are readily soluble in water are described as hydrophilic. Conversely, those that are not readily soluble in water are described as hydrophobic. They are said to be nonpolar and water does not readily dissolve them. The interactions of water with polar and nonpolar molecules are crucial to the function and structure of the lipid-bilayer plasma membrane. Water molecule is the most abundant molecule inside a cell, accounting for about 70% or more of the total cell mass.2 The cell contains water (together with ions, solutes, and other molecules) in its cytosol.
The water content of a human body is referred to as body water. It occurs in the tissues, such as bones, muscles, adipose, blood, and practically everywhere. The average adult human body is about 50 to 60 % water. The body of a newborn Infant contains a higher water percentage (as much as 93% of the bodyweight). The body water in humans (and in animals, too) is contained in various bodily fluids, i.e. extracellular fluid and intracellular fluid.
The extracellular fluid is that which is contained in body areas outside of cells and constitutes 1/3 of the body water. The intracellular fluids are those found inside the cells. They constitute about 2/3 of the body water.
Water on earth is also abundant. It exists as solid, liquid, or gas. About 70% of the Earth’s surface is water. Bodies of water on Earth serve as important habitats for aquatic organisms and a source of water for terrestrial living things. Three major types of aquatic habitats are marine water, freshwater, and brackish water.
Usage of the Term
Apart from being defined as a chemical substance with a chemical formula of H2O, the term may also pertain to a body of water, such as sea, rivers, and lakes, and naturally-occurring water like mineral water. It may also pertain to an aqueous solution of a substance, for example, ammonia water, wastewater. It is also a colloquial term for the amniotic fluid, which is a leakage of fluid prior to or in beginning labor, before the rupture of the amnion.
Try to answer the quiz below to check what you have learned so far about water.
Read:
- Running Water Freshwater Communities – Biology Tutorials
- Abiotic Factors – Water Conditions – Freshwater Ecology
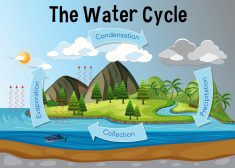
- The Water Cycle
- Plant Water Regulation and Water in Plants – Biology Tutorials
References:
- Rae-Dupree, J. & DuPree, P. (2019, January 1). 4 Types of Chemical Bonds – dummies. Retrieved from ://www.dummies.com/education/science/anatomy/4-types-of-chemical-bonds/ Link
- Cooper, G. M. (2000, January 1). The Molecular Composition of Cells. Retrieved from ://www.ncbi.nlm.nih.gov/books/NBK9879/ Link
© Biology Online. Content provided and moderated by Biology Online Editors