Protein Activity and Cellular Metabolism
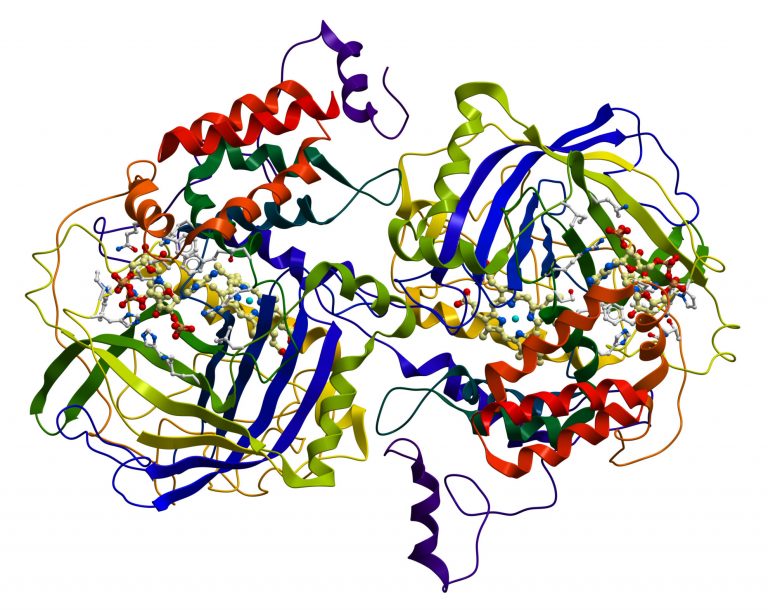
Structural depiction of catalase, an enzyme
Table of Contents
Protein Binding Sites
The ability of various molecules and ions to bind to specific sites on the protein surface forms the basis of the wide variety of protein functions. A ligand is any molecule or ion that is bound to the protein surface by either (1) oppositely charged ionic or polar groups or (2) van der Waals forces between nonpolar regions and a binding site is the protein region where a ligand binds. Chemical specificity and affinity are distinct properties of a binding site.
Chemical specificity
A protein may have several binding sites, each site is specific for a particular or a particular type of ligand. This high chemical specificity is due to the complementary shapes of the ligand and the protein.
Affinity
Affinity is the strength of ligand-protein binding. A binding site could have a high or low affinity for a ligand, depending on the nature of the groups in both and proximity to each other.
Saturation
A single binding site could be occupied or unoccupied and the fraction of sites that are occupied is called saturation. The percent saturation depends on (1) the concentration of unbound ligand and (2) the affinity of the binding site. This saturation will be reflected in the biological activity of the protein-ligand complex
Competition
If there is a competition between two similar ligands for the same binding site, an increase in the concentration of one will inhibit the binding of the other.
Regulation of Binding Sites
Allosteric modulation
Involves a change in the shape of the functional (active) site by the non-covalent binding of a ligand modulator molecule into a regulatory site. The modulation may take the form of either a turn-off or a turn-on function. In a multimeric protein, there can be an increase in the affinity for ligand binding due to cooperativity among the functional groups. This process occurs in the binding of oxygen to hemoglobin.
Covalent modulation
Involves change in the shape of the functional site by the covalent bonding of a chemical group, mostly a phosphate group (PO42-) by a phosphorylation reaction.
Enzymes and Chemical Reactions
Metabolism consists of synthesis (anabolism) and breakdown (catabolism) of organic molecules required for cell structure and function. Chemical reactions involve: (1) the breaking of chemical bonds in reactant molecules and (2) making of new chemical bonds to form product molecules. Energy is either added or released as heat during chemical reactions.
Determinants of reaction rates
Rate of a chemical reaction (number of product molecules formed per unit time) is influenced by:
- Reactant concentration – more the reactants, higher is the rate.
- Activation energy – the energy required by reactant molecules to enter an activated state in which chemical bonds can be broken and formed. The higher the activation energy required, the lower is the reaction rate.
- Temperature – The higher the temperature, the higher is the reaction rate because reactant molecules can acquire the required activation energy.
- Catalyst – a substance that decreases the required activation energy to increase the reaction rate. The chemical composition of a catalyst is not altered by the reaction and thus a single catalyst molecule can be used over and over again.
Reversibility of a reaction
The energy released during a reaction determines the reversibility of a reaction. Greater the energy released during a reaction, smaller is the probability of product molecules obtaining this energy and undergoing the reverse reaction to reform the reactants. In such a case, the ratio of product to reactant concentration will be large and the reaction will tend to be irreversible. In a reversible chemical reaction, the rate of forward reaction decreases and the rate of reverse reaction increases as the reaction progresses until the two are equal in a state called the chemical equilibrium, at which point there is no further change in the concentration of reactants and products.
Law of mass action
Effect of reactant and product concentration on the direction of the net reaction. Increasing the concentration of reactants or decreasing the concentration of products drives the reaction forward and vice versa. This mechanism is important in controlling the direction of metabolic pathways.
Enzymes
Protein catalysts
(A few RNA molecules also possess catalytic activity). In an enzyme-mediated reaction, an enzyme binds to reactants (substrates) to form an enzyme-substrate complex, which breaks down to release products and the enzyme. The region of the enzyme to which the substrate binds is called the active site, the shape of which determines the chemical specificity of the enzyme.
Cofactors
Substances that bind to enzymes to alter their conformations and make them active. Some cofactors are trace elements. In cases where the cofactor is an organic molecule, it is called a coenzyme. Coenzymes are derived from vitamins.
Regulation of enzyme-mediated reactions
Rate of an enzyme-mediated reaction depends on:
- Substrate Concentration: The higher the substrate concentration higher the reaction rate until the enzyme is saturated. Substrate concentration is altered by changes in supply from outside a cell due to changes in diet, rate of absorption from the intestine, changes in the permeability of plasma membrane, or changes in intracellular breakdown and synthesis of the substance.
- Enzyme Concentration: The higher the enzyme concentration, the higher the reaction rate.
- Enzyme Activity: The activity of an enzyme can be altered by allosteric or covalent modulation of the binding site affinity. Modulators are products of other chemical reactions or activated by chemical signals.
Multi-enzyme metabolic pathways
A sequence of enzyme-mediated reactions. Generally consists of a rate-limiting reaction, the slowest step in the sequence that regulates the rate of the whole pathway. The enzyme controlling this step is strongly regulated, often by end-product inhibition, or inhibition by the final product of the pathway.
ATP
Adenosine triphosphate is the primary molecule to which energy is transferred during the breakdown of fuel molecules, carbohydrates, and fats. (A cell cannot use heat energy to perform its functions). ATP is then hydrolyzed to release energy which can be used by the energy-requiring processes in cells such as (1) production of force and movement, (2) active transport across membranes and (2) synthesis of organic molecules used in cell structures and functions. ATP is an energy transfer molecule and NOT an energy storage molecule. It transfers energy from fuel molecules to cells in small amounts.
Metabolic Pathways
Glycolysis
Partially catabolizes carbohydrates, primarily glucose. Converts a 6 C glucose into two 3 C pyruvate. Produces a net gain of 2 ATP by a process called substrate-level phosphorylation. The reactions do not use oxygen and take place in the cytosol. If oxygen is present (aerobic conditions), the pyruvate enters the Krebs cycle. In the absence of oxygen (anaerobic conditions), pyruvate is converted to lactate. Glycolysis occurs in cells lacking mitochondria, e.g., erythrocytes and in certain skeletal muscle cells during intense muscle activity. Other monosaccharides, e.g., fructose and galactose can also be catabolized by glycolysis. In some organisms, e.g., yeast, pyruvate is converted into CO2 and alcohol by a process called fermentation.

Krebs cycle
Also called the citric acid cycle or tricarboxylic cycle. Utilizes molecules produced during carbohydrate, protein or fat breakdown and produces CO2. hydrogen bound to coenzymes and 1 ATP. The reactions occur in the mitochondrial matrix and utilize oxygen. The primary molecule entering the cycle is acetyl coenzyme A (acetyl CoA) derived from pyruvate, fatty acids or amino acids. Total ATP production is 2 since 2 pyruvate molecules are entering the cycle from glycolysis.

Oxidative Phosphorylation
Oxidative phosphorylation uses the hydrogen attached to the coenzymes from the Krebs cycle and oxygen to produce water and 34 ATP molecules, releasing hydrogen-free form of the coenzymes in the process. Occurring in the inner mitochondrial membrane mediated by cytochromes, the process is also called an electron transport chain.

Carbohydrate Synthesis
Glycogen storage
Glucose is stored in skeletal muscles and liver in the form of glycogen, a polysaccharide. Enzymes for glycogen synthesis and breakdown are located in the cytosol. Whether glucose enters the catabolic pathway to form pyruvate or the anabolic pathway to form glycogen is controlled at a single point, working under a feedback mechanism operated by hormones that modulate enzymes.
Glucose synthesis
Glucose is synthesized in liver and kidneys from intermediates derived from the catabolism of glycerol and some amino acids, by a process called gluconeogenesis. The major substrate in this process is pyruvate and 6 ATP molecules are consumed to produce 1 molecule of glucose.
Fat Metabolism
Fat catabolism
A substantial amount of energy is derived from the catabolism of fatty acids that takes place in the mitochondrial matrix, by a series of reactions called beta-oxidation. Acetyl CoA is produced, which can then enter the Krebs cycle. The catabolism of an 18 C saturated fatty acid yields 146 ATP.
Fat synthesis
Fat accounts for a major portion of the energy stored in the body. Acetyl CoA molecules come together to form long, even-numbered C chains and these fatty acids combine with glycerols to form triacylglycerols, in the smooth endoplasmic reticulum.
The majority of body fat is stored in cells called adipocytes, the entire cytoplasm of which is filled with a single fat droplet. Adipocytes synthesize and store triacylglycerols during food uptake and cluster to form adipose tissue, most of which underlies the skin.
Protein and Amino Acid Metabolism
Amino acid catabolism
Protein catabolism requires proteases to break the peptide bonds and release smaller peptides or amino acids. Amino acids can be catabolized either to form ATP or to provide intermediates for a variety of other molecules.
The amino group of amino acid is either removed by oxidative deamination to produce a keto acid and NH3 or transferred to a keto acid by transamination. The keto acid can enter the glycolytic pathway or the synthetic pathways for glucose and fat. The N from the amino group can be used to synthesize important N containing molecules such as purines and pyrimidines.
The toxic NH3 passes into the blood through the plasma membrane and goes to the liver where it is linked with CO2 to form the relatively nontoxic urea, which is excreted by the kidneys.
Amino acid synthesis
Keto acids, e.g., pyruvic acid can be transaminated to form amino acids. This process can only form 11 of the 20 amino acids and the remaining 9, which must be obtained from food, are called essential amino acids.
Total free amino acid pools in the body are derived from:
- Ingested protein degraded to amino acids during digestion,
- Synthesis of nonessential amino acids from keto acids
- Breakdown of body proteins.
These are the pools from which proteins can be synthesized.
Essential nutrients
Essential nutrients are substances that are required for the optimal function of the body but cannot be synthesized at all or can be synthesized only in inadequate amounts by the body. They must be continually supplied in food. Water is an essential nutrient because far more is lost in urine, from skin and respiratory tract than can be synthesized by the body. Mineral elements, 9 essential amino acids, two fatty acids – linoleic and linolenic acids, inositol, choline, and carnitine are essential nutrients.
Vitamins
Vitamins are a group of 14 organic essential nutrients, required in small amounts. Plants and bacteria have enzymes to synthesize vitamins and these are primary sources of vitamins. Excess water-soluble vitamins are excreted through urine while fat-soluble ones are stored in fat tissue.
For practical application on enzymes, this laboratory worksheet will help further understand enzymes.
You will also like...
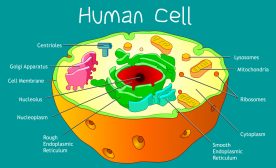
Cell Structure
A typical eukaryotic cell is comprised of cytoplasm with different organelles, such as nucleus, endoplasmic reticulum, G..

Regulation of Biological Systems
Regulation of Biological Systems tutorials are focused on the modulation of biological systems from cell to population l..
Dominance
This tutorial presents Gregor Mendel's law of dominance. Learn more about this form of inheritance and how it can be pre..

Adaptation Tutorial
Adaptation, in biology and ecology, refers to the process or trait through which organisms or the populations in a habit..

Gibberellins and Gibberellic Acid
This tutorial describes the role of gibberellin family in plants. Find out the effects of gibberellin on plant growth an..
Sleep and Dreams – Neurology
While learning and intelligence are associated with the functions of a conscious mind, sleep and dreams are activities o..
